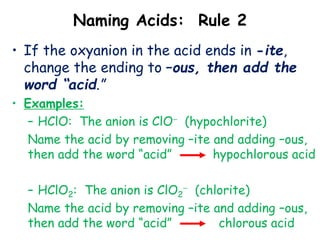
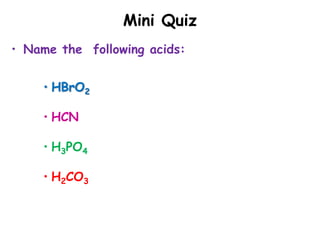
This document discusses the nomenclature (naming) of acids. It provides three rules for naming acids based on whether they contain oxygen or not. For acids containing oxyanions, the ending is changed from "-ate" to "-ic" or from "-ite" to "-ous" before adding "acid". For acids containing monatomic or polyatomic anions without oxygen, the ending is changed from "-ide" to "-ic" and "hydro-" is prefixed before adding "acid". Examples of applying each rule are given.