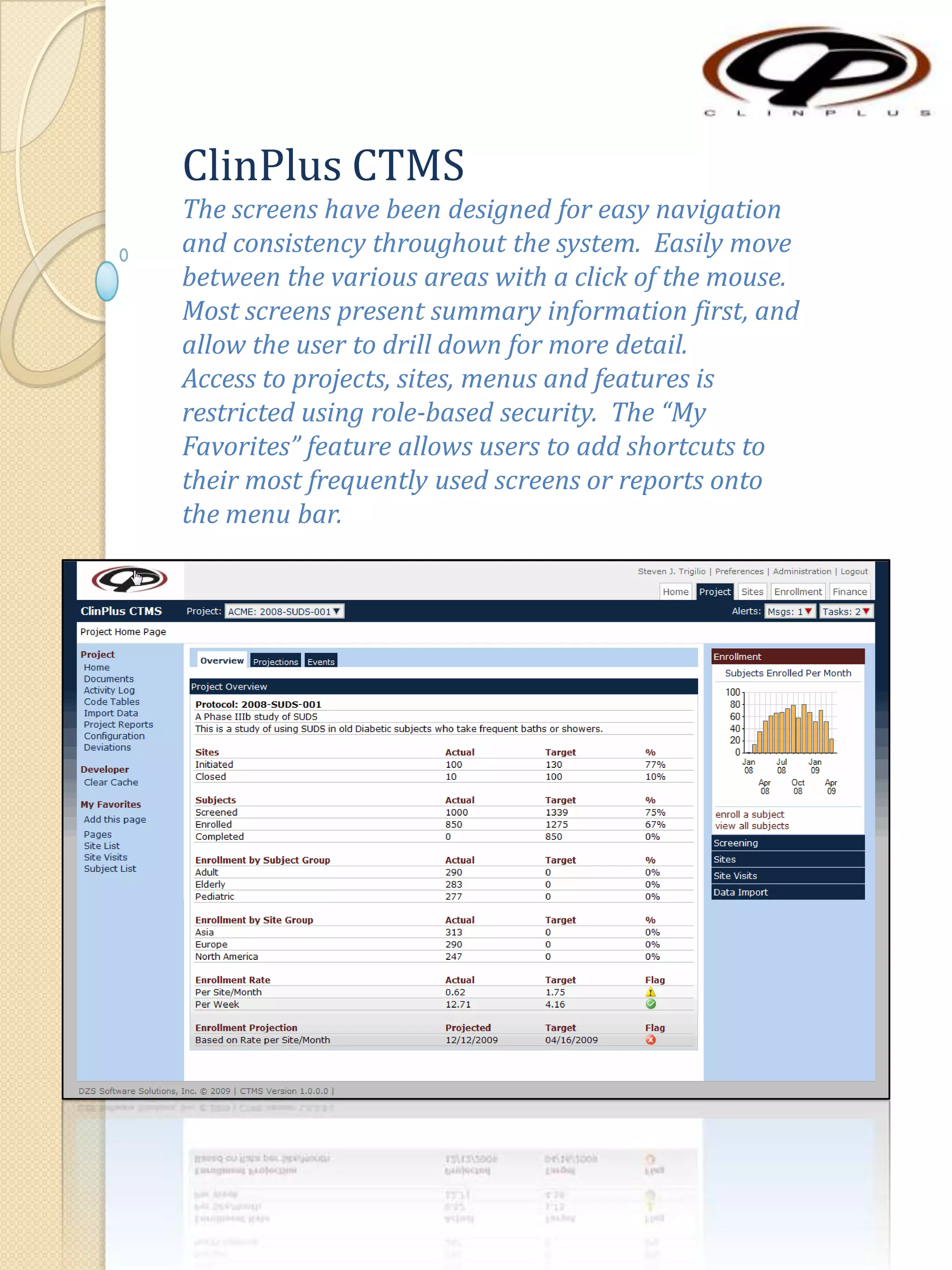
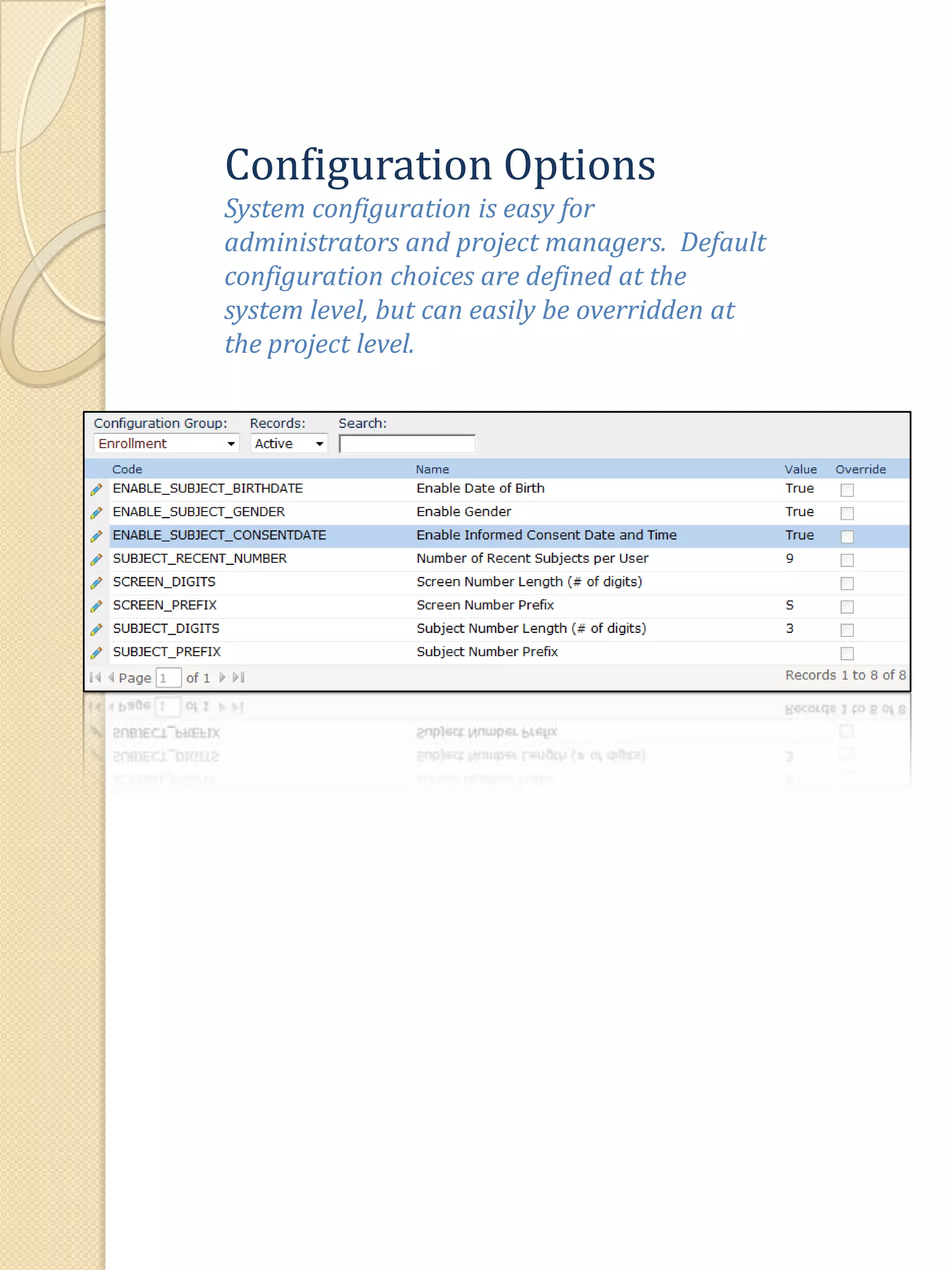
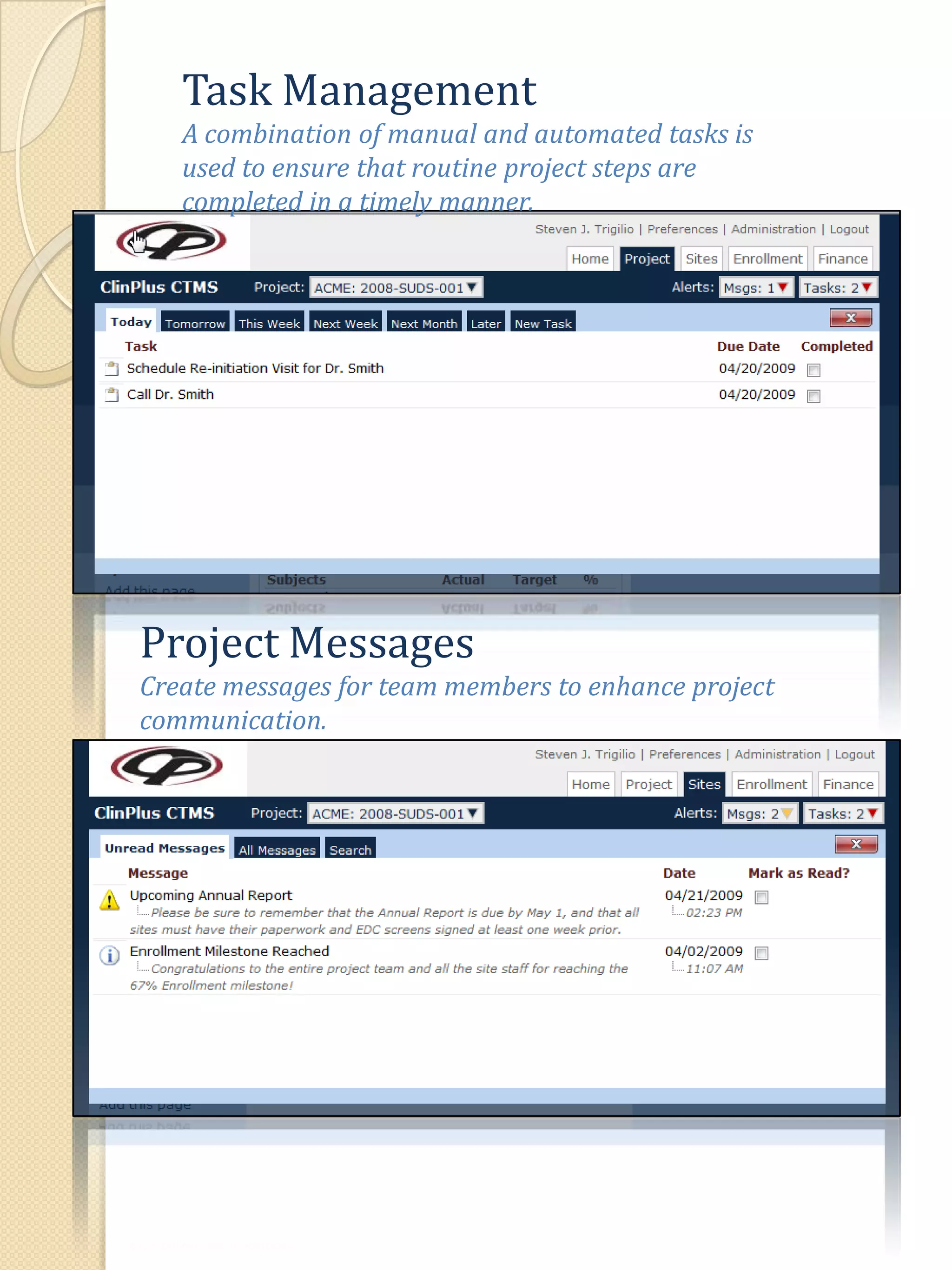
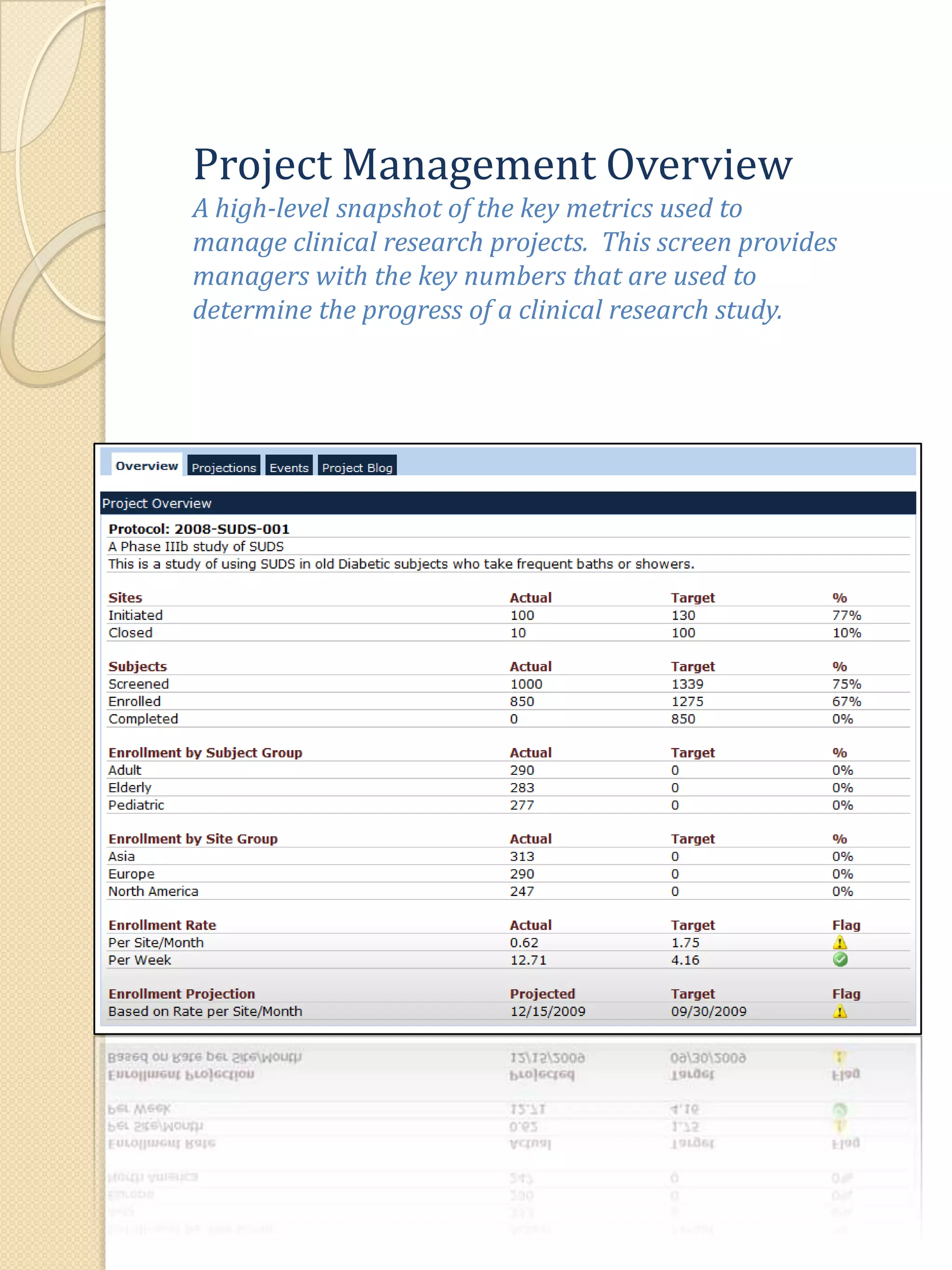
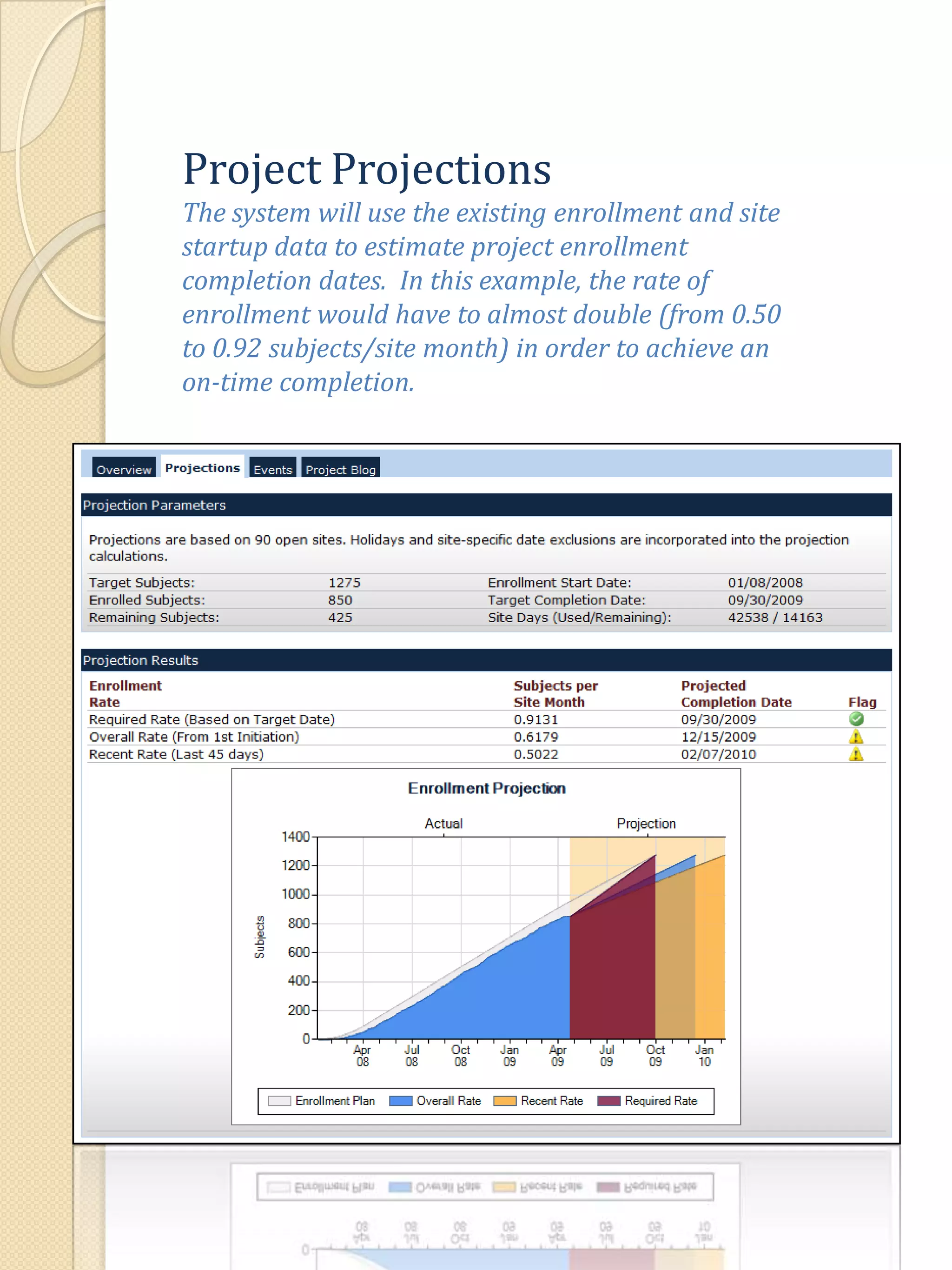
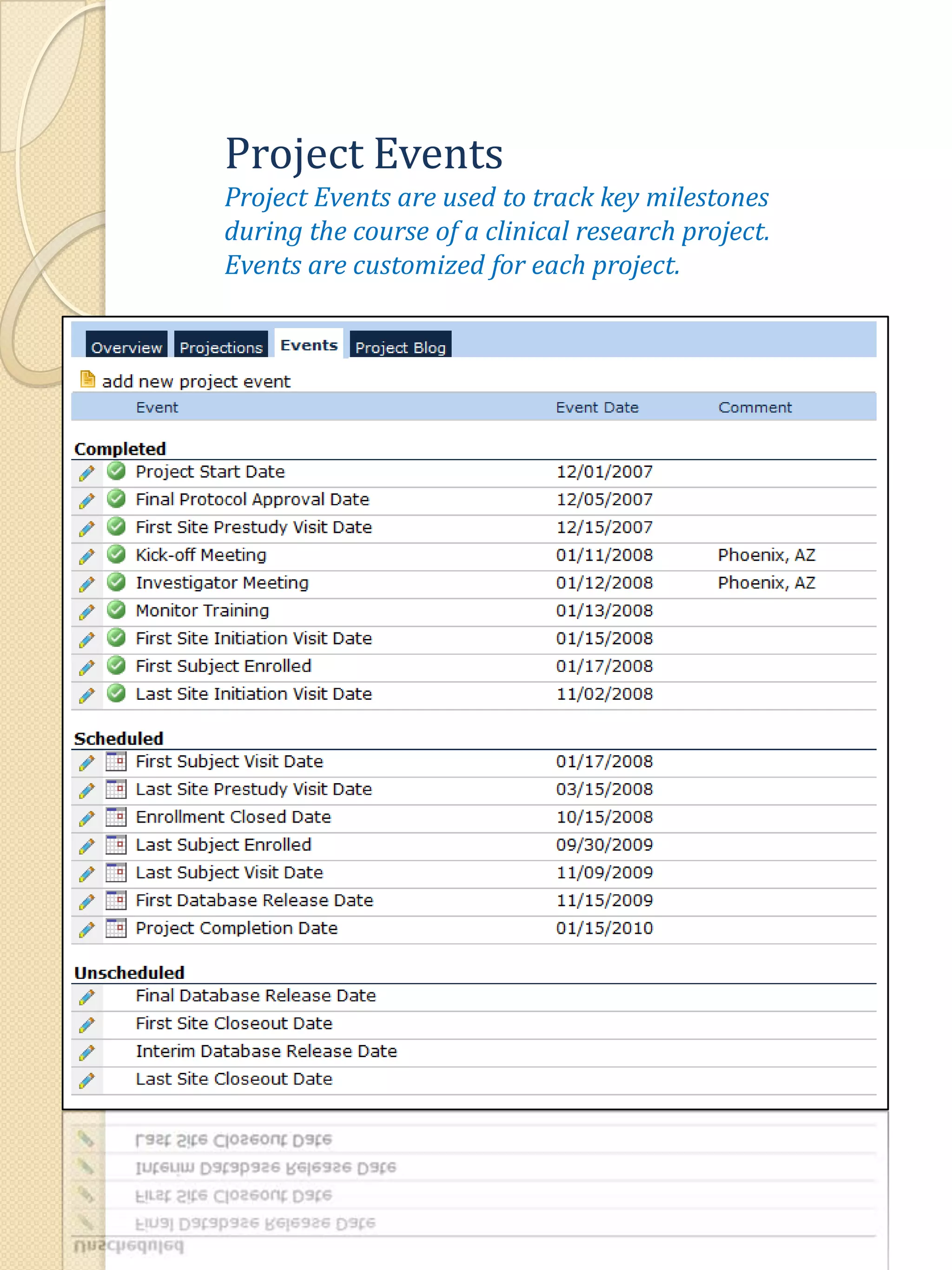
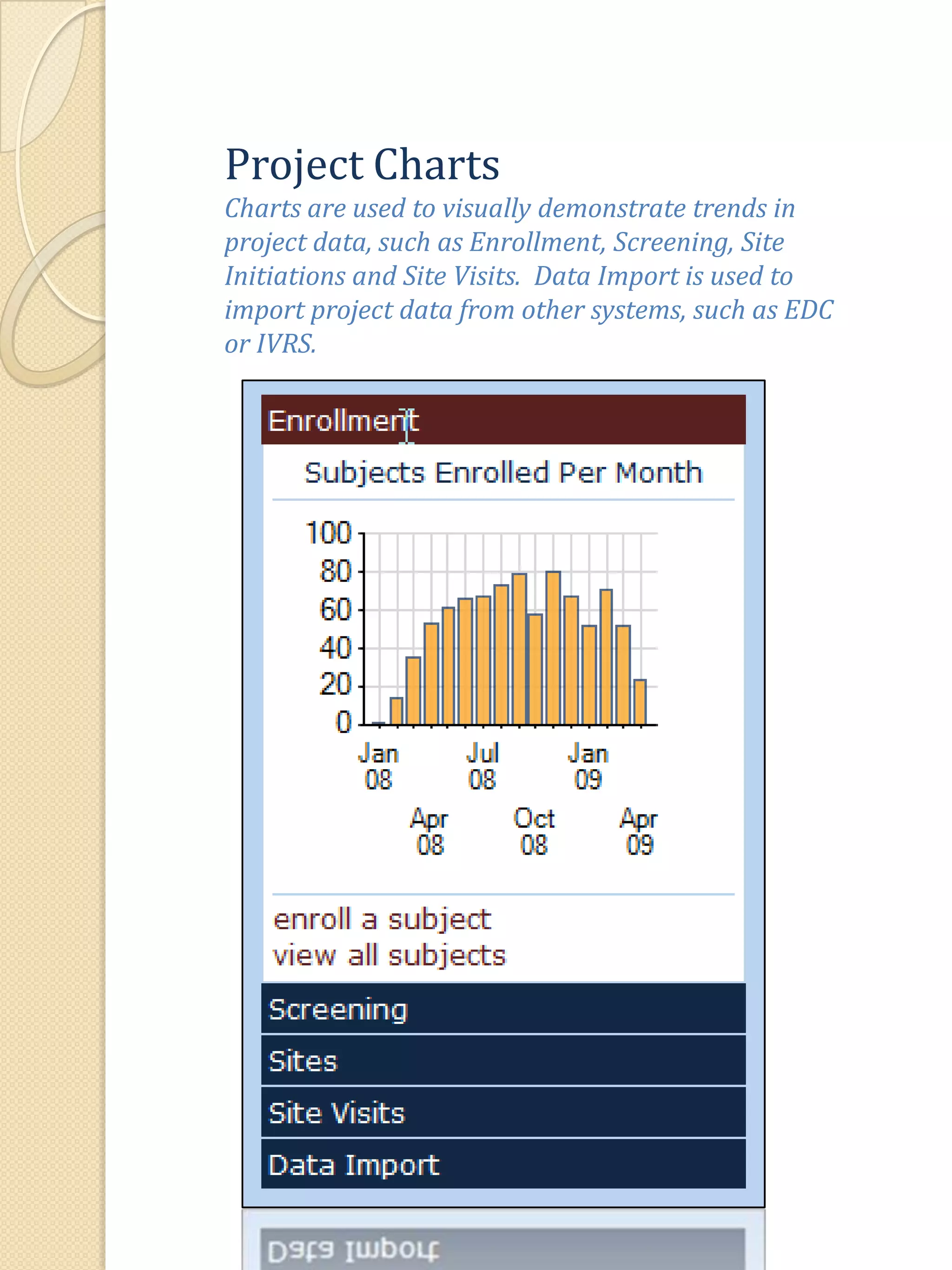
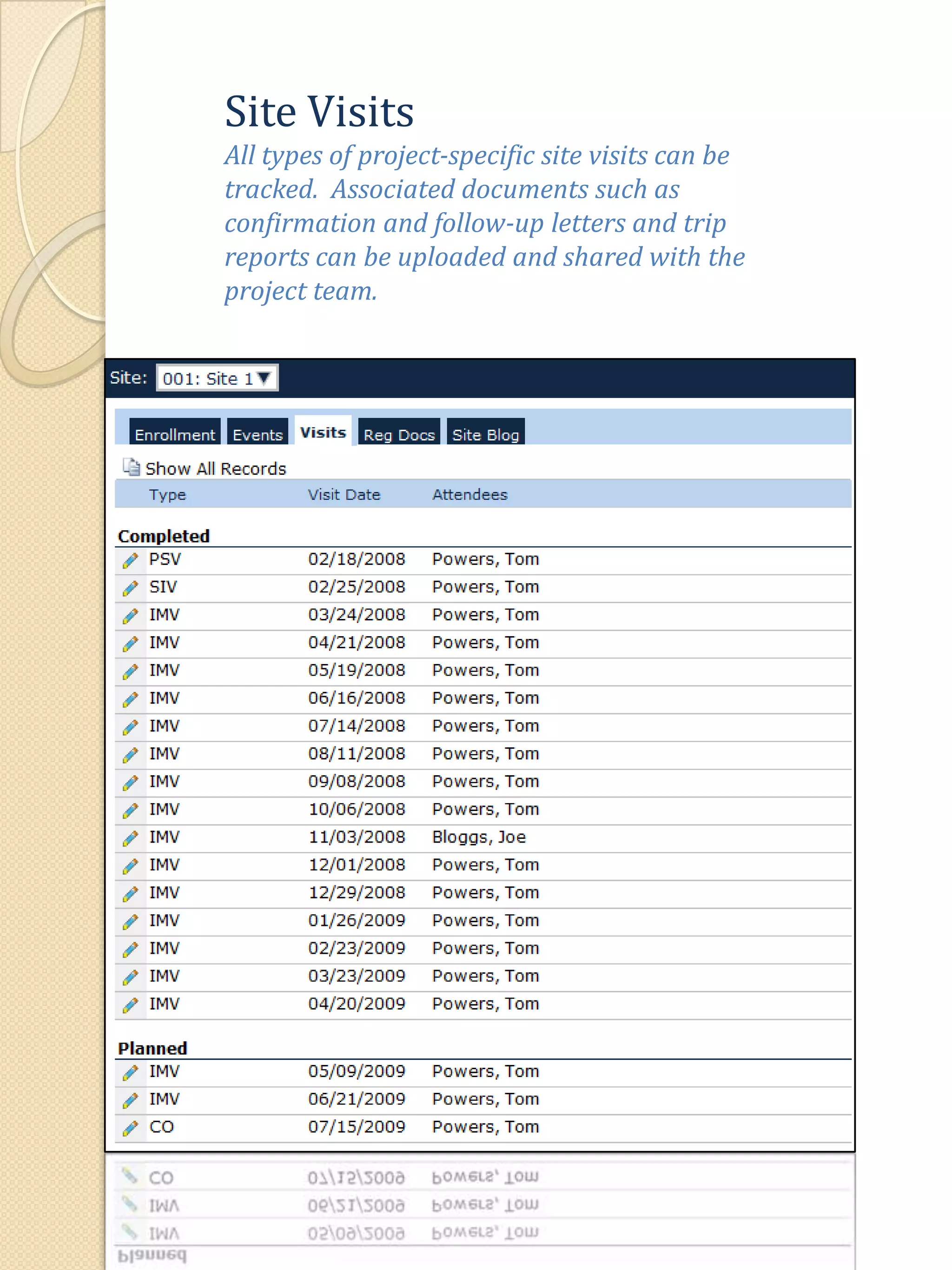
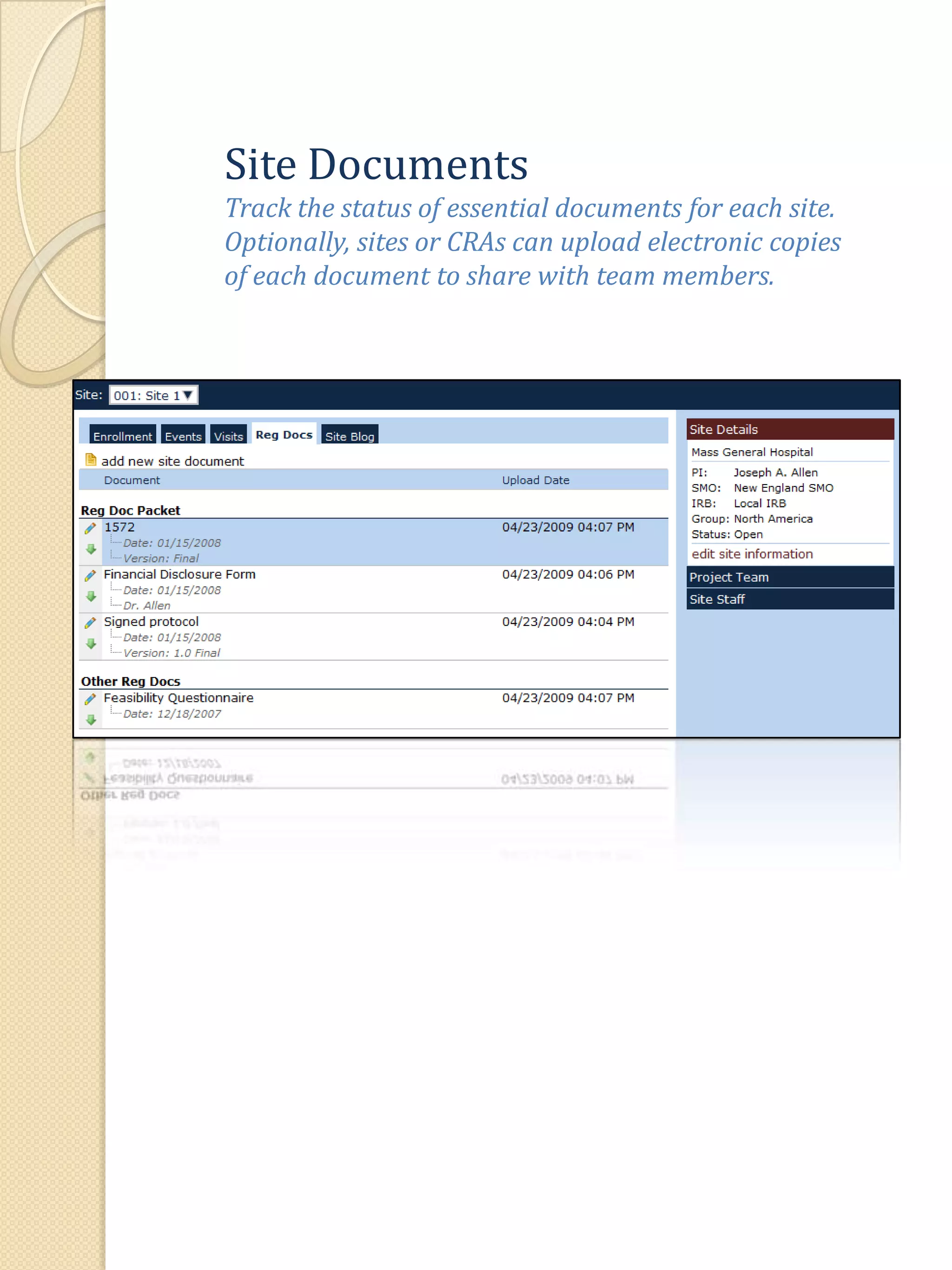
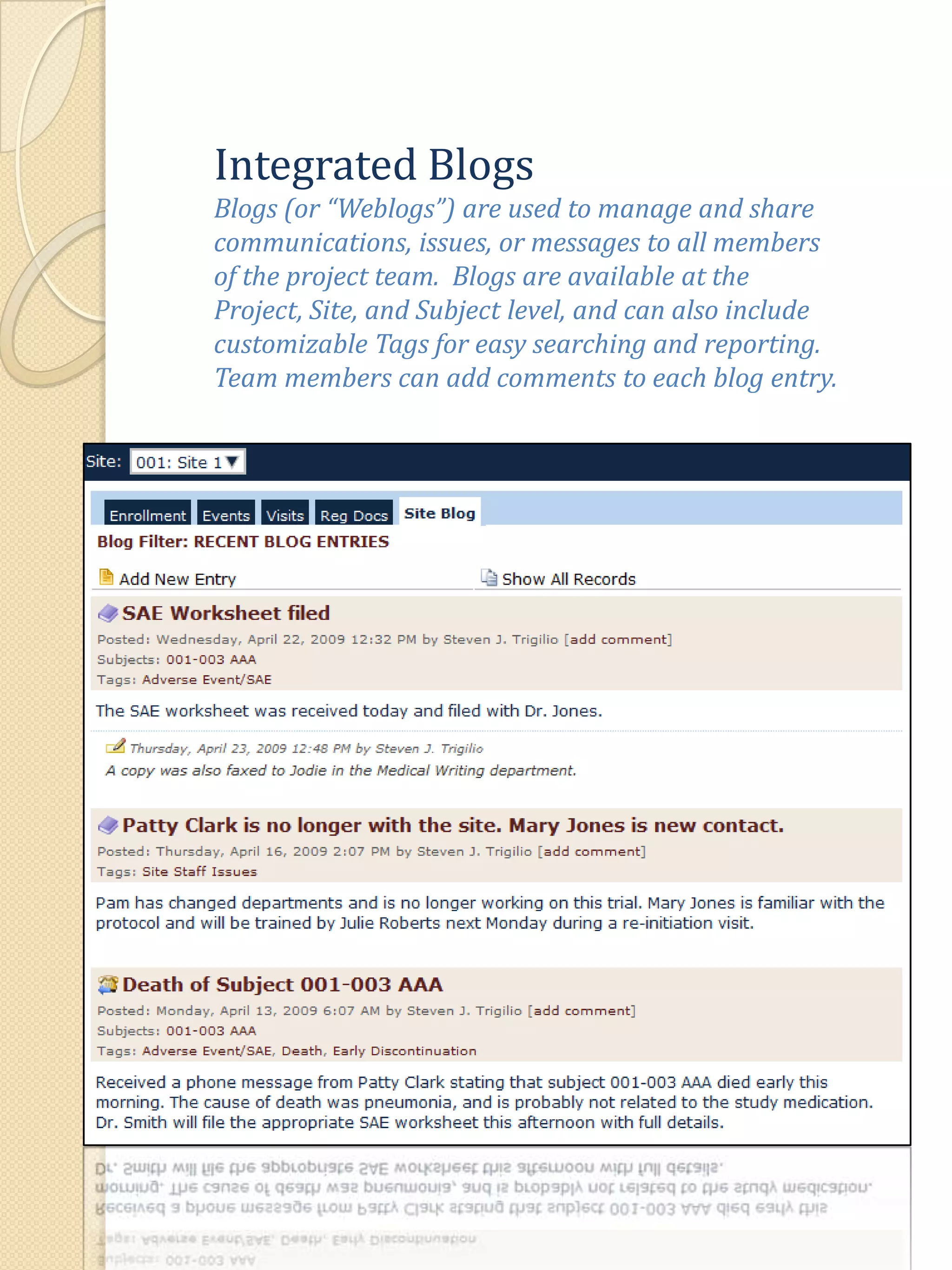
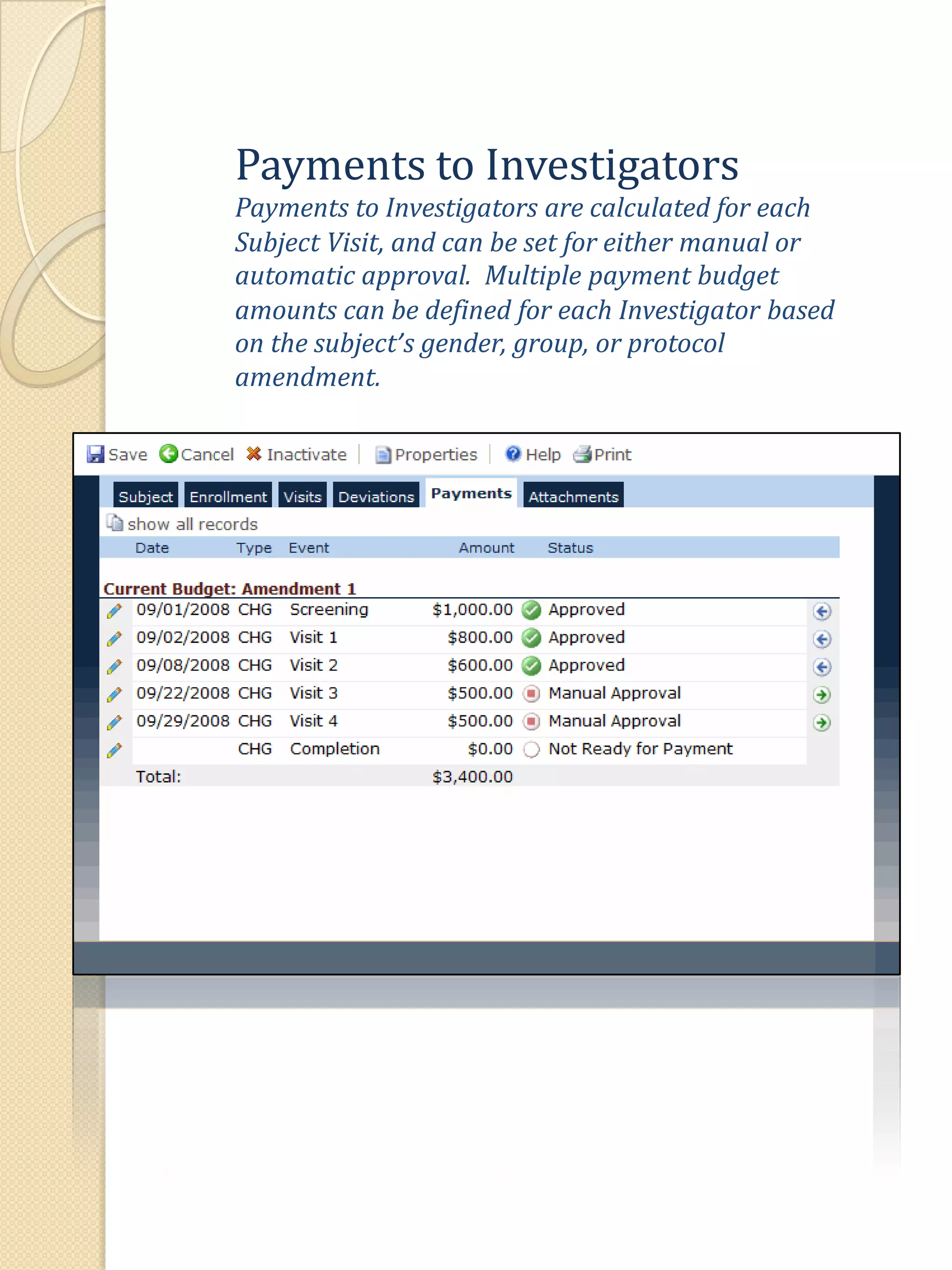
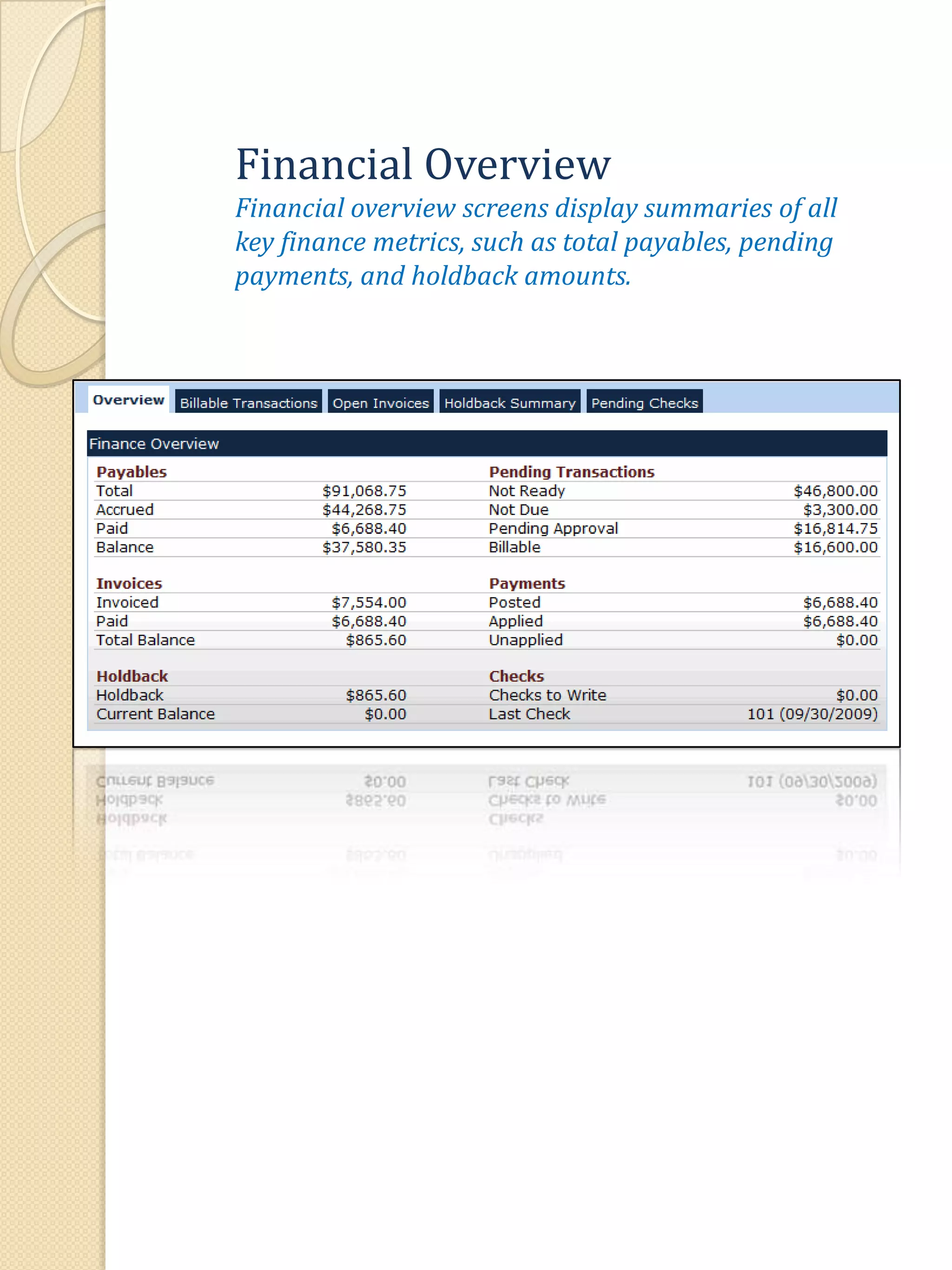
The document describes various features of the ClinPlus CTMS clinical trial management system. It allows for easy navigation between screens that present summary information first with the ability to drill down for details. Role-based security restricts access to projects, sites, menus and features. Configuration can be defined at the system level but overridden at the project level. A combination of manual and automated tasks are used to ensure project steps are completed on time. Project messages, overviews, projections, events, charts, site events, visits, documents, blogs, payments to investigators, and financial overviews are described as key features to manage clinical research projects and metrics.