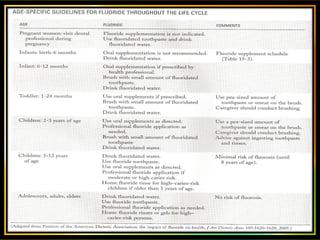
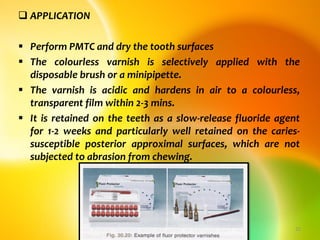
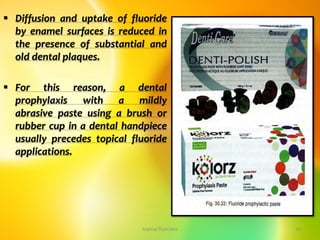
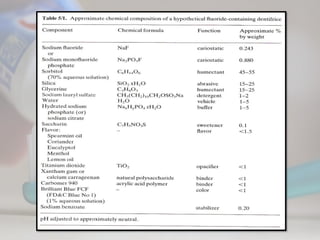
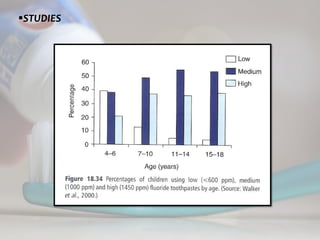
This document discusses various professionally and self-applied topical fluorides. It describes the composition and application techniques of sodium fluoride, stannous fluoride, acidulated phosphate fluoride, fluoride varnishes including Duraphat and Fluor Protector, and fluoride-releasing dental materials. It also covers self-applied topical fluorides such as toothpastes, mouthwashes, and gels. Topical fluorides strengthen enamel and make it more resistant to acid attacks from plaque, helping prevent and control dental caries.



























![ Prophylaxis of enamel surfaces results in removal of a
superficial layer of enamel as well as the pellicle (Vrbic et al.,
1967).
It has been mentioned that surface enamel contains higher
levels of fluoride than is found in internal layers, therefore, a
prophylaxis removes a fluoride-rich layer.
If prophylaxis pastes containing fluoride are used, the lost
fluoride is replenished and there is a small, but significant,
net gain in the concentration of fluoride [Steams, 1973]
topical fluorides 34](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-34-320.jpg)
![ Fluoride-containing prophylactic pastes currently widely
used in North America include APF-containing pastes which
contain silicon dioxide, or zirconium silicate or insoluble
sodium metaphosphate as the abrasive material [Clarkson
and Wei, 1982].
Mixing topical fluoride solution such as APF with flour of
pumice for use as a paste is not recommended. The pumice
binds and, therefore, inactivates the fluoride and raises the
pH thereby causing a reduction in fluoride uptake by
enamel.
topical fluorides 35](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-35-320.jpg)



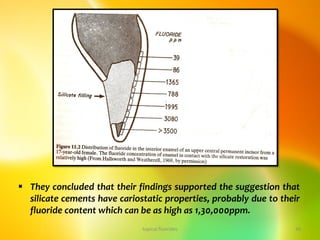
























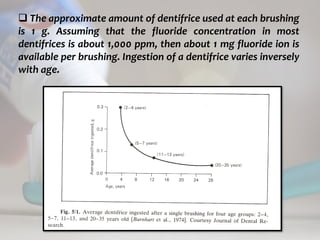
![topical fluorides 74
Ericsson and Forsman [1969] found that children aged 4-5
years who used 0.5 g of dentifrice per brushing retained
(presumably ingested) from 26 to 33% while a 6- to 7- year
group retained 25-28%.
This corresponds to a retention of about 0.12 mg fluoride per
brushing or 0.25 mg/day.
In a group of children (5-7 years) who used 1.0 g dentifrice
per brushing. Barnhart et al. [1974] found that about 0.5 mg
fluoride was ingested with two brushings.](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-74-320.jpg)


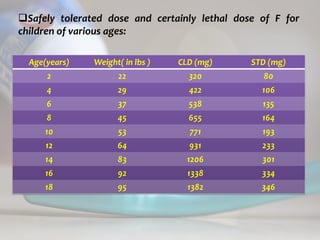







![ c
topical fluorides 85
A 0.2% sodium fluoride solution to be used weekly or
fortnightly is similarly prepared except that 2 g of sodium
fluoride are weighed and distilled water added to make up I
liter.
There are also numerous commercial preparations designed
for use by families at home or for use by large numbers of
school children in supervised mouthrinse programs in schools.
The estimated annual cost of a sodium fluoride mouthrinse
program in school is approximately $0.75 (US) per student,
assuming that each student rinses 36 times in a school year
[Wei, 1982].](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-85-320.jpg)
![ c
topical fluorides 86
The supervised use of
fluoride rinses is
recommended as an
effective caries-reducing
regimen for school children
over the age of 5 years.
The method is simple,
inexpensive and safe if used
as directed.
A guide for implementing
self-applied fluoride in
schools has been proposed
[US National Institute for
Dental Research, 1977].](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-86-320.jpg)

![ c
topical fluorides 88
FREQUENCY OF RINSING
There is some clinical support for the proposition that
frequent use of low levels of fluoride is more cariostatic than
less frequent use of formulations containing higher
concentrations of fluoride.
What is clear is that a schedule of monthly rinsing is not as
effective as rinsing daily, weekly or fortnightly [Toren and
Ericsson, 1974; Birkeland and Toren, 1978].](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-88-320.jpg)

![ c
topical fluorides 90
INGESTION OF MOUTHRINSES
Both Hellstrom [1960] and Ericsson and Eorsnum [1969]
reported that fluoride retention after the use of mouthrinses in
children age 6 and over was about 20%.
Wei and Kanellis [1983] confirmed the work of previous
investigators that preschool children usually swallow as much
as 30-40% of the rinse.
The younger the age group of greater the percentage of the
rinse swallowed.](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-90-320.jpg)
![ c
topical fluorides 91
Ericsson and Forsman [1969] also found that below the age of
4, children often cannot control their swallowing reflexes and
may consistently swallow 100% of the rinse.
Such children, of whatever age, should be excluded from
fluoride rinse programs in order to avoid excessive systemic
intake. The volume of the mouthrinse and its duration have also
been shown to influence the degree of retention.
Birkeland [1973] found that in older children significantly more
fluoride was ingested when using 10 than 7 ml of mouthrinse.
Similarly Birkeland and Lokken [1972] showed that adults
swallowed more mouthrinse when longer rinsing times were
used.](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-91-320.jpg)












![ c
topical fluorides 106
Several brands of fluoridated wooden toothpicks (TePe [TePe,
Malmo, Sweden], Butler [Butler, Chicago, IL], Elmex, and Jordan
[Jordan, Oslo, Norway]) and dental tape and floss (Johnson &
Johnson [Johnson & Johnson, New Brunswick, NJ], Butler,
Elmex, Oral-B [Gilette, Boston, MA] and Jordan) have recently
been introduced.](https://image.slidesharecdn.com/14-topicalfluorides-part2advanced-230822131012-91823f70/85/14-topical-fluorides-part-2-advanced-pdf-106-320.jpg)