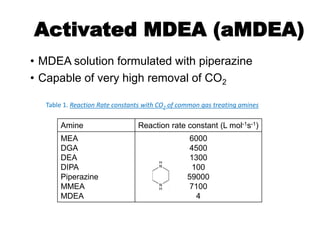
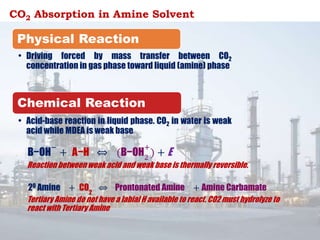
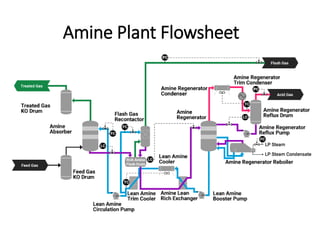
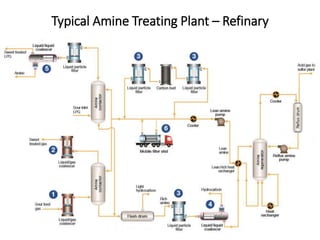
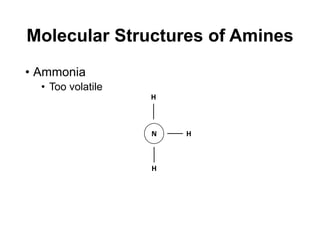
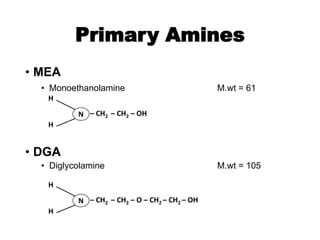
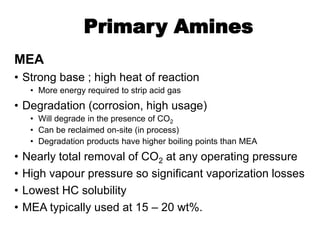
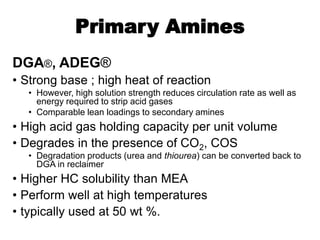
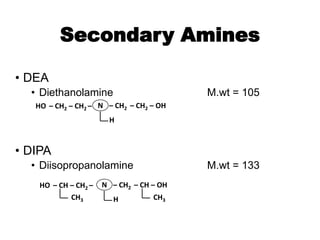
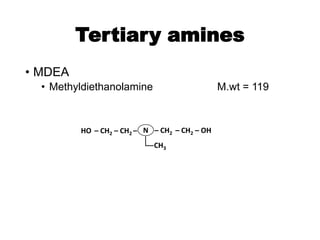
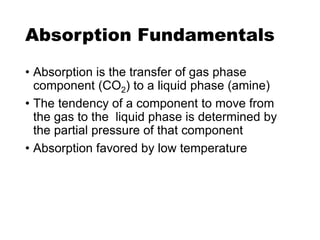
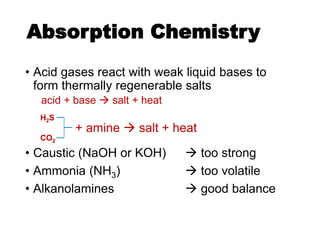
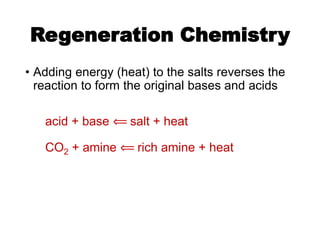
The document discusses amine treating processes for gas plants and refineries, outlining topics such as equipment, operations, chemistry, and the various types of amines used. It details the purpose of amine plants, including CO2 recovery and emissions minimization, as well as the chemical reactions involved in CO2 and H2S removal. Additionally, it covers the characteristics and efficiencies of different amine types, emphasizing the importance of reaction rates and conditions in optimizing gas treatment operations.



















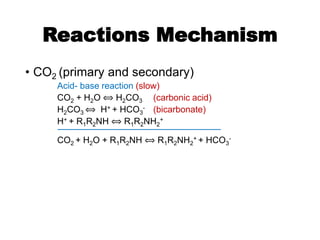
![Reaction Mechanism
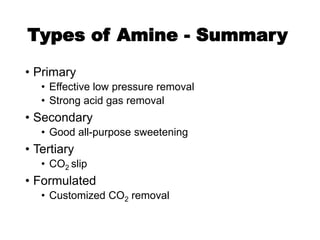
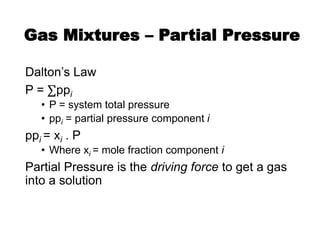
• CO2 reactions (primary and secondary amines)
– carbamate formation
2 [R1R2NH] + CO2 ⟺ [R1R2NH]H+ + [R1R2N-COO]–
(fast)
“carbamate reaction”](https://image.slidesharecdn.com/1-240710024047-370f709e/85/1-Process-Gas-Swwetening-Principles-pptx-45-320.jpg)
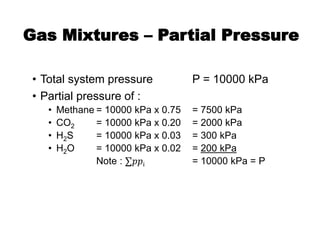
![Reactions Mechanism
CO2 reactions
• Tertiary Amines
CO2 + H2O ⟺ H2CO3
(slow)
[R1R2NR3] + H2CO3 ⟺ [R1R2NR3]H+ + HCO3
-
(fast)](https://image.slidesharecdn.com/1-240710024047-370f709e/85/1-Process-Gas-Swwetening-Principles-pptx-47-320.jpg)