This document provides information about a mathematics exam paper that will take place on April 10, 2014 and has a moderate to tough difficulty level. The paper will consist of 60 multiple choice questions (MCQ) and short answer type questions (SCQ) across various topics in fundamentals of mathematics including quadratic equations. The questions are divided into 10 sets with varying number of MCQ and SCQ in each set. The total marks for the paper are 210.
![-1
MATHEMATICS PAPER : CT-1
TARGET DATE : 10-04-2014
PAPER LEVEL : MODERATE TO TOUGH
SYLLABUS : Fundamentals of Mathematics-I, Quadratic Equation
S.No. Subject Nature of Questions No. of Questions Marks Negative Total
1 to 10 SCQ 10 3 –1 30
11 to 15 MCQ 5 4 0 20
16 to 20 Integer (double digits) 5 4 0 20
21 to 30 SCQ 10 3 –1 30
31 to 35 MCQ 5 4 0 20
36 to 40 Integer (double digits) 5 4 0 20
41 to 50 SCQ 10 3 –1 30
51 to 55 MCQ 5 4 0 20
56 to 60 Integer (double digits) 5 4 0 20
60 210
Paper-1 CT-1
Total Total
Maths
Physics
Chemistry
SCQ
1. Let n be an integer greater than 1 and let an =
n
1
log 1001
. If b = a3 + a4 + a5 + a6 and [BALG]
c = a11 + a12 + a13 + a14 + a15. Then value of (b – c) is equal to
ekukn, 1 lscM+hiw.kkZad la[;kgSrFkkekukan =
n
1
log 1001
;fn b = a3 + a4 + a5 + a6 vkSj c = a11 + a12 + a13 +
a14 + a15 rc (b – c) dkeku cjkcj gS-
(A) 1001 (B) 1002 (C) – 2 (D*) –1
Sol. an = log1001n
b = log10013 × 4 × 5 × 6 and c = log1001(11×12×13×14×15)
(b – c) = log1001
3 4 5 6
11 12 13 14 15
= log1001(1001)
–1
= –1
2. The number of integral values of 'a' for which both roots of the equation x
2
– 2x – a
2
= 0 lie between the
roots of equation x
2
– 2x + a
2
–11a + 12 = 0, is [QELR]
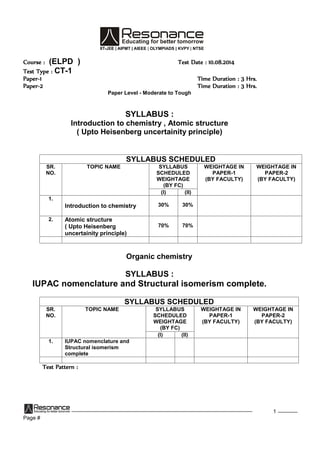
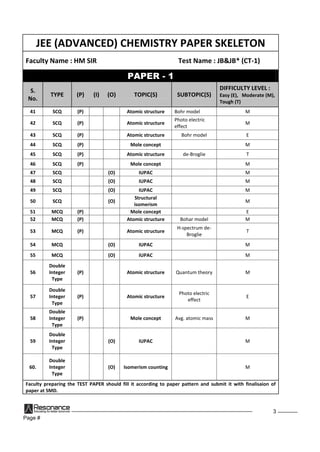
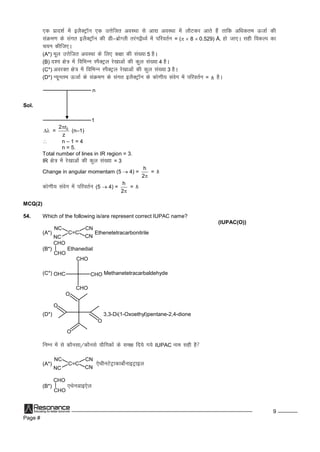
'a' dsiw.kkZad ekuksadhla[;kftldsfy, lehdj.kx
2
– 2x – a
2
= 0 dsnksuksaewy lehdj.kx
2
– 2x + a
2
–11a + 12 = 0 es
ewyksadse/; fLFkr gS-
(A) 0 (B) 1 (C*) 2 (D) 3
Sol. Let be the root of x
2
– 2x – a
2
= 0
ekuk lehdj.kx
2
– 2x – a
2
= 0 dsewy gS
2
– 2 = a
2
and roots of x
2
– 2x – a
2
= 0 lie between roots of x
2
– 2x + a
2
– 11a + 12 = 0
rFkkx
2
– 2x – a
2
= 0 ds ewy lehdj.kx
2
– 2x + a
2
– 11a + 12 = 0 dsewyksdse/; fLFkr gS
2
– 2 + a
2
– 11a + 12 < 0
2
– 2 = a
2
a
2
+ a
2
– 11a + 12 < 0
2a
2
– 11a + 12 < 0
(2a – 3)(a – 4) < 0
a
3
, 4
2
number of integral values of 'a' is = 2
'a' dsiw.kkZad ekuksadhla[;k= 2 gS](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/75/1-ct-1-paper-1-10-aug-2014-1-2048.jpg)
![-2
3. If x = 2 + 3i, then value of x
4
– x
3
+ 10x
2
+ 3x – 5 is equal to [BAGQ]
;fn x = 2 + 3i rc x
4
– x
3
+ 10x
2
+ 3x – 5 dkeku cjkcj gS-
(A) 127 (B) 122 (C) 120 (D*) – 122
Sol. x = 2 + 3i
(x – 2)
2
= – 9 x
2
– 4x + 13 = 0
x
4
– x
3
+ 10x
2
+ 3x – 5 = (x
2
– 4x + 13)(x
2
+ 3x + 9) – 122
= – 122
4. The complete solution set of inequation
x 2
2
(e – 2)(x – 5x 4)
(x – 5x 6)
0, is [BAIR]
vlfedk
x 2
2
(e – 2)(x – 5x 4)
(x – 5x 6)
0 dklEiw.kZgy leqPp; gS&
(A) (–, –1] [n 2,2) (3, 4] (B) (–,n 2] (1, 2) (3, 4]
(C*) (–,n 2] [1, 2) (3, 4] (D) [n 2, 1] (2, 3) [4, )
Sol.
x
(e – 2)(x – 1)(x – 4)
(x – 2)(x – 3)
0
x (–,n 2] [1, 2) (3, 4]
5. Product of the roots of the equation 2
2
1
2 x
x
– 9
1
x
x
+ 14 = 0, is
lehdj.k 2
2
1
2 x
x
– 9
1
x
x
+ 14 = 0 dsewyksadkxq.kuQy gS-
(A) 5 (B) 2 (C) 10 (D*) 1
Sol. Given equation become nhxbZlehdj.k
2x
4
– 9x
3
+ 14x
2
– 9x + 2 = 0
product of roots ewyksadkxq.kuQy = 1
Alter
Let ekukx +
1
x
= t
2
2 t – 2 – 9t + 14 = 0 2t
2
– 9t + 10 = 0
t = 2,
5
2
x +
1
x
= 2 or x +
1
x
=
5
2
x = 1, x = 1 or x = 2,
1
2
product of roots = 1
ewyksadkxq.kuQy = 1
6. The number of integral values of x satisfying
2 2
x x x x 1
4 3.2 4
is [QEMS]
2 2
x x x x 1
4 3.2 4
dkslarq"V djusokysx dsiw.kkZad ekuksadhla[;kgS-
(A) 5 (B) 3 (C*) 4 (D) 2
Sol.
2 2
x x x x 1
4 3.2 4
2 2
x x x x 1
4 – 3.2 – 4 0
2 2
2x x x 2x 2
2 – 3.2 – 2 0
2 2 2
2x x x x x 2x 2
2 – 4.2 2 – 2 0
2 2 2
x x x x x x 2
2 (2 – 4.2 ) 2 (2 – 2 ) 0
](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-2-320.jpg)
![-3
2 2
x x x x
(2 – 4.2 )(2 2 ) 0
2
x x
(2 – 4.2 ) 0
2
x x
2 4.2
x
2
x + 2 x
2
– x – 2 0
(x – 2)(x + 1) 0 x [–1, 2] 4 integers iw.kk±d
7. Complete set of real values of k for which kx – x
2
+ 9 – x < 0 x R is [QEGR]
(A) (0, ) (B) k (–, ) (C) k (–, –1] (D*) no such real k exists
k dsokLrfod ekuksadklEiw.kZleqPp; ftldsfy, kx – x
2
+ 9 – x < 0 x R gS-
(A) (0, ) (B) k (–, )
(C) k (–, –1] (D*) k dkdksbZokLrfod eku fo|eku ugh
Sol. Roots are real as product of roots negative
ewy okLrfod gSrFkkewyksadkxq.ku_ .kkRed gSA
Alter
– x
2
+ x(k – 1) + 9 < 0
D < 0
(k – 1)
2
– 4 × (–1)(+9) < 0
(k – 1)
2
+ 36 < 0
No real k exists
k dkdksbZokLrfod eku fo|eku ugha
8. If the equation (a
2
+ a – 30)x
2
+ (b – 1)(a
2
–2a – 15)x + (b
2
– 6b + 5) = 0 has more than two different
solutions for x, then number of possible ordered pairs (a, b) is [QEGR]
;fn lehdj.k(a
2
+ a – 30)x
2
+ (b – 1)(a
2
–2a – 15)x + (b
2
– 6b + 5) = 0 dsx dsfy, nkslsvf/kd gy j[krsgS
rc (a, b) dslaHkkfor Øfer ;qXeksadhla[;kgS-
(A) 1 (B) 2 (C*) 3 (D) 4
Sol. for more than two different solution, the equation must be an identity and for that
nkslsvf/kd gy dsfy, lehdj.k,d loZlfedkgksxh
a
2
+ a – 30 = 0 and vkSj (b – 1)(a
2
– 2a – 15) = 0 and vkSj(b
2
– 6b + 5) = 0
a = –6, a = 5 and vkSj b = 1, a = 5, a = –3 and vkSjb = 1, b = 5
possible ordered pairs (a, b) are
lefor Øfer ;qXe (a, b) gS
(5, 1), (5, 5), (–6, 1)
three possible ordered pairs (a, b) are there.
rhu laHkkfor Øfer ;qXe (a, b) gS
9. If , , are the roots of the equation 2x
3
– 7x
2
+ 3x – 1 = 0, then the value of (1 –
2
)(1 –
2
)(1 –
2
) is
;fn lehdj.k2x
3
– 7x
2
+ 3x – 1 = 0 dsewy , , gSrks(1 –
2
)(1 –
2
)(1 –
2
) dkeku gS- [QETE]
(A) 39 (B) – 39 (C)
39
4
(D*) –
39
4
Sol. 2x
3
– 7x
2
+ 3x – 1 = 0 b
g
2x
3
– 7x
2
+ 3x – 1= 2(x – )(x – )(x – ) ……..(i)
(i) put x = 1 j[kusij, we get
2(1 – )(1 – )(1 – ) = – 3 ……….(ii)
(ii) put x = – 1 in (1) esaj[kusij, we get
2(–1 – )(–1 – )(–1 – ) = –13 2(1 + )(1 + )(1 + ) = 13 ………(iii)
multiply (2) and (3), we get
4(1 –
2
)(1 –
2
)(1 –
2
) = –39
10. The complete solution set of the inequation
1/ 2
1
log | x |
> 1 is [BAMS]](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-3-320.jpg)
![-4
vlfedk
1/ 2
1
log | x |
> 1 dklEiw.kZgy leqPp; gS-
(A)
1
–2, –
2
1
, 1
2
(1, 2) (B)
1
–2, –
2
1
, 2
2
(C*) (–2, – 1)
1
–1, –
2
1
, 1
2
(1, 2) (D)
Sol.
1/ 2
1
log | x |
> 1
1/ 2log | x | < 1 but ijUrq log1/2|x| 0
–1 < log1/2|x| < 1 but ijUrq|x| 1 x 1, – 1
2 > |x| >
1
2
1
2
< |x| < 2
x (–2, – 1)
1
–1, –
2
1
, 1
2
(1, 2)
MCQ
11. If range of expression
2
x – 12
2x – 7
(x R) is (–, a] [b, ) and let the solution of the equation
a alog b log x 3
a.x b b is x = c, then [QEGR]
(A) (a + b) and c are both prime (B*) (a + b) and c are coprime number
(C*) (a + b + c) is a perfect square (D) a + b = c
;fn O;atd
2
x – 12
2x – 7
(x R) dkifjlj(–, a] [b, ) gSrFkklehdj.k a alog b log x 3
a.x b b dkgy x = c gSrc -
(A) (a + b) vkSjc nksuksavHkkT; gSA (B*) (a + b) rFkkc lgvHkkT; la[;kgSA
(C*) (a + b + c) ,d iw.kZoxZgSA (D) a + b = c
Sol. Let ekuk y =
2
x – 12
2x – 7
x
2
– 2xy + 7y – 12 = 0
D 0
4y
2
– 4 × 1 × (7y – 12) 0
y
2
– 7y + 12 0
y (–, 3] [4, )
a = 3 and vkSjb = 4
equation becomes 3 3log 4 log x
3.x 4 64
lehdj.k 3 3log 4 log x
3.x 4 64 ls
3 3log x log x
3(4 ) 4 64
3log x
4 16 3log x = 2
x = 9 = c
12. Let a and b are the solutions of the equation 4 1/ 41 log x –1 log x 26
5 5
5
such that a > b, then the value of
a
b
is
(A*) an even number (B*) a rational number [BALG]
(C*) a composite number (D) a prime number
ekuka vkSj b lehdj.k 4 1/ 41 log x –1 log x 26
5 5
5
dsgy bl izdkj gSfd a > b rc
a
b
dkeku gS–](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-4-320.jpg)
![-5
(A*) le la[;k (B*) ifjes; la[;k
(C*) la;qDr la[;k (D) vHkkT; la[;k
Sol. 4 4log x –log x–1 26
5.5 5 .5
5
Let ekuk 4log x
5 = t
5t +
1
5t
=
26
5
25t
2
– 26t + 1 = 0
t = 1 or t =
1
25
log4x = 0 or ;k log4x = – 2
x = 1 or ;k x =
1
16
a
b
= 16
13. If ax
2
+ bx + c = 0 has imaginary roots and a,b,c R, then which of the following options are CORRECT?
;fn ax
2
+ bx + c = 0 dsdkYifud ewy gSrFkka,b,c R, rc fuEu esalsdkSulsfodYi lghgS? [QEGR]
(A) ax
2
+ bx + c > 0 x R (B*) a(a – b + c) > 0
(C*) a(ax
2
+ bx + c) > 0 x R (D*) a
2
+ c
2
+ 2ac > b
2
Sol. D < 0
(ax
2
+ bx + c) will be of same sign as that of 'a'
(ax
2
+ bx + c) 'a' dsleku fpUg dkgksxk
C is correct lghgS
(B) Let ekukf(x) = ax
2
+ bx + c
D < 0
Two graphs are possible nksvkj[kslaHko gS
–1
a > 0 and rFkkf(–1) > 0 a – b + c > 0
a(a – b + c) > 0
–1
a < 0 and rFkk f(–1) < 0 a – b + c < 0
a(a – b + c) > 0
(D) a
2
+ c
2
+ 2ac – b
2
> 0
(a + c)
2
– b
2
> 0
(a – b + c)(a + b + c) > 0
f(–1) f(1) > 0 true lR; see the above two graphs nksvkjs[kmij fn[kk;svuqlkj
14. The value of
2 2
nb
a b
( na)( nb)
6a log b log a
e
is [BALG]
(A*) independent of a (B*) independent of b (C) dependent on a (D) dependent of b
2 2
nb
a b
( na)( nb)
6a log b log a
e
dkeku gS-
(A*) a lsLora=k (B*) b lsLora=k (C) a ij fuHkZj (D) b ij fuHkZj](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-5-320.jpg)
![-6
Sol.
2 2
nb
a b
na nb
6a log b log a
e
=
nb
a b
nb
1 1
6.a . log b. log a
2 2
a
=
6
4
=
3
2
15. Identify which of the following statement(s) are 'CORRECT' ? [QEGR]
(A*) For ax
2
+ bx + c = 0, (a 0) if 4a + 2b + c = 0, then roots are 2 and
c
2a
.
(B) If is repeated root of ax
2
+ bx + c = 0, (a 0) then ax
2
+ bx + c = (x – )
2
.
(C*) For ax
2
+ bx + c = 0, (a 0, a,b,c Q) imaginary roots occur in conjugate pair only.
(D*) If f(x) = ax
2
+ bx + c, (a 0) has finite maximum value and both roots of f(x) = 0 are of opposite sign,
then f(0) > 0.
fuEu esalsdkSulkdFku lghgS?
(A*) ax
2
+ bx + c = 0, (a 0) dsfy, ;fn 4a + 2b + c = 0 rc ewy 2 vkSj
c
2a
gSA
(B) ;fn , ax
2
+ bx + c = 0, (a 0) dkiqujko`fÙkewy gS]rc ax
2
+ bx + c = (x – )
2
.
(C*) ax
2
+ bx + c = 0, (a 0, a,b,c Q) dsfy, dkYifud ewy dsoy la;qXehgksrsgSA
(D*) ;fn f(x) = ax
2
+ bx + c, (a 0) fu;r vf/kdre eku j[krkgSrFkkf(x) = 0 dsnksuksaewy foijhr fpUg dsfpUg gS]
rc f(0) > 0.
Sol. (A) 4a + 2b + c = 0 ax
2
+ bx + c = 0 has one root as 2
other root will be
c
2a
(B) ax
2
+ bx + c = a(x – )
2
(C) coefficients are rational coefficients are real.
imaginary roots occur in conjugate pair
(D) f(0) > 0
Integer Type
16. If x R then absolute difference between the maximum and minimum values of the expression
2
2
x 14x 9
x 2x 3
is [QEGR]
;fn x R rc O;atd
2
2
x 14x 9
x 2x 3
dsvf/kdre vkSj U;wure ekuksadkfujis{kvUrj gS&
Ans. 09
Sol. Let ekuky =
2
2
x 14x 9
x 2x 3
x
2
(y – 1)+ 2x(y – 7) + 3(y – 3) = 0 ………….(i)
Case-1 : If y = 1, then equation (i) becomes
–12x – 6 = 0 x =
1
2
which is real tksfd okLrfod gSA
y = 1 is possible laHko gSA
Case-2 : If y 1, then D 0
4(y – 7)
2
– 4(y – 1).3(y – 3) 0
y
2
– 14y + 49 – 3(y
2
– 4y + 3) 0
–2y
2
– 2y + 40 0
y
2
+ y – 20 0
–5 y 4
Absolute difference = 9
fujis{kvUrj = 9](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-6-320.jpg)
![-7
17. If a,b R, then the smallest natural number 'b' for which the equation x
2
+ 2(a + b)x + (a – b + 8) = 0 has
unequal real roots for all a R, is [QENR]
;fn a,b R, rc U;wure izkd`r la[;k'b' ftldsfy, lehdj.kx
2
+ 2(a + b)x + (a – b + 8) = 0 dslHkha R ds
fy, vleku okLrfod ewy gS&
Ans. 05
Sol. D = 4(a + b)
2
– 4 × 1 × (a – b + 8)
= 4[a
2
+ 2ab + b
2
– a + b – 8]
= 4[a
2
+ a(2b – 1) + (b
2
+ b– 8)]
for unequal real root for all a R
lHkha R dsfy, lHkhvleku okLrfod ewy gS
D > 0 a R
(2b – 1)
2
– 4 × 1 × (b
2
+ b – 8) < 0
4b
2
– 4b + 1– 4b
2
– 4b + 32 < 0
– 8b + 33 < 0
8b > 33
b >
33
8
smallest natural value of b is = 5
b dkU;wure izkd`r eku = 5
18. Let the product of all the solutions of the equation 33 3
3 x 3(log 3x log 3x)log x = 2 be k, then find the
value of 18k. [BALG]
ekuklehdj.k 33 3
3 x 3(log 3x log 3x)log x = 2 dslHkhgyksadkxq.kuQy k gSrc 18k dkeku Kkr dhft,
Ans. 02
Sol. squaring we get
oxZdjusij
1/ 3
1/ 3 3
3
3
log (3x)
log (3x)
log x
3log3x = 4
Let ekuklog3x = t
1
3
(t 1)(t 1)
t
.3t = 4
t + 1 = ±2
x = 3 or x =
1
27
product of roots xq.kuQy dsewy =
1
9
= k
18k = 2
19. If a, b and c are real numbers such that a2 + 2b = 7, b2 + 4c = –7 and c2 + 6a = –14, then find the value of
2 2 2
a b c
2
. [BAMS]
;fn a, b vkSj c okLrfod la[;kbl izdkj gSfd a2 + 2b = 7, b2 + 4c = –7 vkSjc2 + 6a = –14 gS]rks
2 2 2
a b c
2
dkeku Kkr dhft,A
Ans. 07
Sol. a2 + b2 + c2 + 6a + 4c + 2b = – 14
(a + 3)2 + (b + 1)2 + (c + 2)2 = 0
a = – 3, b = – 1 and vkSj c = – 2
so blfy,
2 2 2
a b c
2
=
9 1 4
2
= 7](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-7-320.jpg)
![-8
20. Find the number of integral values of 'm' less than 50, so that the roots of the quadratic equation
mx
2
+ (2m – 1)x + (m – 2) = 0 are rational. [BAMS]
50 lsNksVsm dsiw.kk±d ekuksadhla[;kKkr dhft, tcfd f}?kkr lehdj.kmx
2
+ (2m – 1)x + (m – 2) = 0 dsewy
ifjes; gSA
Ans.06
Sol. D = 4m + 1
now for roots to be rational must be perfect square of a rational number
but since m is an integer it will be perfect square of an integer.
so let blfy, ekuk4m + 1 = k
2
, k
m =
2
k – 1
4
for m to be an integer k must be odd
k = ±1, ±3, ±5, ±7, ±9, ±11, ±13
but ijUrqm 0 There are 6 possible integral values of m.](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-8-320.jpg)
![Page No.-1
JEE – ADVANCED (CT – 1)
Date : - 10/8/2014
Test Syllabus : Mathematical Tools, Rectilinear Motion, Projectile Motion & Relative Motion complete
S.No. Subject Nature of Questions No. of Questions Marks Negative Total
1 to 10 SCQ 10 3 –1 30
11 to 15 MCQ 5 4 0 20
16 to 20 Integer (double digits) 5 4 0 20
21 to 30 SCQ 10 3 –1 30
31 to 35 MCQ 5 4 0 20
36 to 40 Integer (double digits) 5 4 0 20
41 to 50 SCQ 10 3 –1 30
51 to 55 MCQ 5 4 0 20
56 to 60 Integer (double digits) 5 4 0 20
60 210
Paper-1 CT-1
Total Total
Maths
Physics
Chemistry
PAPER-1
SECTION-1 : (Only One option correct type)
[k.M–1 : (d soy ,d lghfod Yi çd kj)
This section contains 10 multiple choice questions. Each question has four choices (A), (B), (C) and
(D) out of which ONLY ONE is correct.
bl [k.M esa10 cgqfod Yi ç'u gSA çR;sd ç'u esapkj fod Yi (A), (B), (C) vkSj (D) gS] ft uesalsd soy ,d
lghgSA
SCQ_(10)
21. If the tangent on the curve 2
y kx (where k is a constant) at x = 1makes an angle 45o
with +x-axis,
then the value of k is
;fn oØ 2
y kx (;gkWk ,d fu;rkad gS) ij x = 1 ij [khaphxbZLi'kZjs[kk+x v{kls45º dks.kcukrhgksrksk dk
eku gksxkA
(A*)
1
2
(B)
1
4
(C) 2 (D) 4
Soln. (A) 2 45ody
k x tan
dx
At x = 1 ij 2k(1) = 1
1
2
k
22. It is given that A R B
and R A
. Then the angle between A & B
is
A R B
rFkkR A
fn;kgqvkgS]rksA & B
dse/; dks.kgksxkA
(A) 1 A
cos
R
(B) 1 B
cos
A
(C*) 1 R
tan
A
(D) 1 R
sin
A
Soln. (C)](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-9-320.jpg)



![Page No.-5
Soln. (A)
30 60
30 60
o o
o o
u sin gt u sin gt
u cos u cos
3 1
u
t
g
30. A particle is thrown vertically upwards with initial speed u m/s (with respect to lift) inside a lift moving
downwards with constant velocity. Its time of flight is T sec. Again the particle is thrown vertically
upwards with same initial speed u m/s. (with respect to lift) inside a lift moving upwards with constant
acceleration g/2, then new time of flight is
uhpsdhrjQ fu;r osx lsxfr'khy fy¶V dsvUnj ,d d.kdksfy¶V dslkis{kÅ /okZ/kj Å ij dhrjQ izkjfEHkd
pky u m/s lsiz{ksfir fd;ktkrkgSA bl le; bldkmMM~;u dky T lSd.M gSA vc nwckjkÅ ij dhrjQ fu;r
Roj.kg/2 lsxfr'khy fy¶V dsvUnj d.kdksfy¶V dslkis{kÅ /okZ/kj Å ij dhrjQ leku izkjfEHkd pky u m/s
lsiz{ksfir fd;ktk;srksbl d.kdku;kmM~M;u dky gksxkA
(A)
2
T
sec (B) 2T sec (C)
3
2
T
sec (D*)
2
3
T
sec
Soln. (D)
2u
T
g
2
2
' u
T
g g /
2 2
3
u
g
2
3
T
SECTION-2 : (One or more option correct type)
[k.M–2 : (,d ;kvf/kd lghfod Yi çd kj)
This section contains 5 multiple choice questions. Each question has four choices (A), (B), (C) and
(D) out of which ONE or MORE are correct.
bl [k.M esa5 cgqfod Yi ç'u gSA çR;sd ç'u esapkj fod Yi (A), (B), (C) vkSj (D) gS] ft uesalsd soy ,d ;k
vf/kd lghgSA
MCQ._(5)
31. A particle is projected at an angle with the horizontal from a point O on a plane which is inclined at an
angle to the horizontal. The particle is moving horizontally when it strikes the plane at a point A.
,d d.kdksfcUnqO ls{kSfrt ls dks.kij urry ij iz{ksfir fd;ktkrkgSA urry dk{kSfrt lsurdks.k gSA
;fn urry dhfcUnqA ij d.kdsVdjkrsle; ;g {kSfrt fn'kkesaxfr'khy gksrks
Fixed
A
B
O
u](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-13-320.jpg)
![Page No.-6
(A) Time of flight
2usin
T
g
sec
mMM~;u dky
2usin
T
g
lSd.M gSA
(B*) Maximum height of the particle
2 2
2
u sin
g
m
d.kdhvf/kdre~~Å WpkbZ
2 2
2
u sin
g
ehVjgSA
(C*) Horizontal range OB =
2
u sin cos
g
m
{kSfrt ijkl OB =
2
u sin cos
g
ehVj gSA
(D*) 1
2tan ( tan )
Soln. (B, C, D)
usin
T
g
2u sin ( )
gcos
tan = 2 tan , 1
2tan ( tan )
2 2
2
max
u sin
H
g
OB (u cos ) (T) =
2
u sin cos
g
32. The vectors A
is given by 2ˆ ˆ ˆA ti (sin t) j t k
where t is time. Then which of the following is (are)
correct ?
lfn'k A
fuEu }kjkfn;ktkrkgSA
2ˆ ˆ ˆA ti (sin t) j t k
tgkWt le; gS]rksfuEu lsdkSulsfodYi lghgS?
(A*) 1 2A (at t )
(B*) 1 2
dA ˆ ˆ ˆ(at t ) i j k
dt
(C*)
2
1 2 1
dA
A (at t )
dt
(D*) 1 3
dA
A (at t )
dt
(A) 1 2
A (t )ij (B) 1 2
dA ˆ ˆ ˆ(t ) i j k
dt
ij
(C) 2
1 2 1
dA
A (t )
dt
ij (D) 1 3
dA
A (t )
dt
ij](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-14-320.jpg)
![Page No.-7
Soln. (A, B, C, D)
2ˆ ˆ ˆA t i sin t j t k
1 ˆ ˆ ˆA(t ) i sin t j k
2A
2
dA ˆ ˆ ˆi cos t j tk
dt
1 2
dA ˆ ˆ ˆ(t ) (i j k)
dt
2
dA ˆ ˆ ˆ ˆ ˆA (i k) (i j) k)
dt
ˆ ˆ ˆ( i j k)
2
2 1
dA
A
dt
1 2 3
dA
A.
dt
33. A car is travelling north along a straight road at 50 km hr –1
. An instrument in the car indicates that the
wind is directed towards east. If car’s speed is 80 km hr–1
, then instrument indicates that the wind is
directed towards south – east. Then angle made by wind’s direction is given by. [RL - TD]
,d dkj lh/khlM+d ij mÙkj fn'kkdsvuqfn'k50 km hr –1
dhpky lsxfr'khy gSA dkj esayxkgqvk;a=kiznf'kZr
djrkgSfd gokiwoZfn'kkdhrjQ izokfgr gSA ;fn dkj dhpky 80 km hr–1
gksrks;a=kgokdhfn'kknf{k.k&iwoZ
dhrjQ iznf'kZr djrkgSrksgokdhfn'kk}kjkcuk;kx;kdks.kgksxkA
(A) 1 3
tan Nof E
5
(B*) 1 5
tan Nof E
3
(C) 1 1
tan Nof W
2
(D) = tan–1
(5) N of E
(A) 1 3
tan
5
iwoZlsmÙkj dhrjQ (B*) 1 5
tan
3
iwoZlsmÙkj dhrjQ
(C) 1 1
tan
2
if'pe lsmÙkj dhrjQ (D) = tan–1
(5) iwoZlsmÙkj dhrjQ
Sol. (B)
WGV
= x y
ˆ ˆV i V j
1WCV
is towards east
1WCV
iwoZdhrjQ
Vx
Vy
50
Vy
= 50 m/s ; Vx
> 0
2WCV
is towards south-east](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-15-320.jpg)

![Page No.-9
(C) velocity of man is 10 m/s. (D) drift of man in the direction of flow is 600 m.
,d O;fDr ikuhdslkis{kpky hr/kmjˆ6iˆ3V1 lsrSj ldrkgSA ;fn ikuh hr/kmiˆ5V2 pky lsizokfgr
gSA ;fn unhdhpkSM+kbZ jˆm500d gksrks
(A*) O;fDr dkiFkljy js[kh; gSA (B*) unhdksikj djusesayxkle; 5 min gSA
(C) O;fDr dkosx 10 m/s gSA (D) izokg dhfn'kkesaO;fDr dkfoLFkkiu 600 m gSA
Soln: (A, B)
Resultant path of man is straight line.
O;fDr dkifj.kkehiFkljy js[kh; gSA
hr/kmjˆ6iˆ8VVV RMRM
s/m
9
25
hr/km10VM
min5
1000
60
6
500
t
Drift fopyu m
3
2000
1000
60
5
8 .
SECTION-3 : (Integer value correct Type)
[k.M – 3 : (iw.kk±d eku lghçd kj)
This section contains 5 questions. The answer to each question is a Two digit integer, ranging from
00 to 99 (both inclusive).
bl [k.M esa5 ç'u gSaA çR;sd ç'u dkmÙkj 00 ls99 rd ¼nksuksa'kkfey½dschp dknksvad ksaokykiw.kk±d gSA
Integer_(5)_(Double Digit)
36. A ball is projected with speed 10 m/s from ground at angle 300 with the vertical. After some time it
again fall on the ground, then the magnitude of average velocity of the ball in this interval (in m/s) is
,d xsan dkstehu ls10 m/s dhpky lsÅ /okZ/kj ls30° dks.kij iz{ksfir fd;ktkrkgSA dqN le; i'pkr~;g
okil tehu ij fxjrhgS]rksbl le;kUrjky esaxsan dkvkSlr osx dkifjek.k(m/s esa) Kkr dhft,A
Ans. 05
Soln : (5)
o
avgV ucos 10cos60 5m / s
37. If c,b,a are three vectors having magnitudes 1, 2, 3 respectively such that a b c 0
then value of
a.cc.bb.a is :
;fn c,b,a rhu lfn'kksadkifjek.kØe'k%1, 2, 3 bl izdkj gSfd a b c 0
gksrks a.cc.bb.a dkeku
Kkr dhft, :
Ans. 07
Soln : (7)
0cba
0a.cc.bb.a2cba 222
2
cba
a.cc.bb.a
222
](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-17-320.jpg)



![Page #
4
Physical paper-1
SCQ(6)
41. The ratio of area of 4
th
circular orbit of He+ ion to that of 3
rd
orbit of Li2+ ion is : (ATS(P))
He+ vk;u d h 4
th
o Li2+ v k;u d h 3
rd
o`Ùkkd kj d {kk d s{ks=kQ y d k vuqikr fuEu gS&
(A*) 64 : 9 (B) 4 : 3 (C) 8 : 3 (D) 3 : 4
Sol.
4 2
A B
4 2
B A
n Z
n Z
=
4 2
4 2
4 3
3 2
=
64
9
2
2 n
area r , r
Z
2
2 n
r , r
Z
{ks=kQy
42. Light of wavelength 2 falls on a metal having work function hc/0. Photoelectric effect will take
place only if: (ATS(P))
d k;Z Q y u hc/0 ;qDr ,d /kkrq ij 2rjax}S/;Z d k izd k'k vkifrr gksrk gSA izd k'k fo| qr
izHkko d soy rHkh mRiUu gksxk t c%
(A) 0 (B) 20 (C*) 2 0 (D) 0 / 2
Sol.
43. An electron in a hydrogen like species jumps from an energy level to another energy level in such
a way that its kinetic energy changes from 'a' to
a
4
. The change in total energy of electron will be :
,d gkbMªkstu leku Lih'kht esa,d bysDVªkWu ,d Å tkZLrj lsvU; Å tkZLrj esabl izdkj LFkkukUrfjr
gksrkgS]fd bldhxfrt Å tkZ'a' ls
a
4
rd ifjofrZr gksrhgS]rksbldhdqy Å tkZesaifjorZu gksxk:
(ATS(P))
(A*) +
3
4
a (B) –
3
8
a (C) +
3
2
a (D) –
3
4
a
Sol. Change in total energy =
a
– – ( a)
4
dqy Å tkZesaifjorZu =
a
– – ( a)
4
=
3
a
4
44. LPG contains n-butane and isobutane. Mass of carbon in 14.5 Kg of LPG.
LPG n-C;wVsu o vkblksC;wVsu j[krkgSA LPG ds14.5 Kg esadkcZu dknzO;eku fuEu gS&
(Mole-1(P))
(A) 3 Kg (B*) 12 Kg (C) 14.5 Kg (D) 10 Kg
Sol. mass of Carbon dkcZu dknzO;eku =
14.5
48
58
= 12 Kg
45. Calculate ratio of de-Broglie wavelength of O2 molecule to He atom if ratio of their kinetic energy
is 1 : 18.
O2 v.kqo He ijek.kqdh Mh&czksXyh rjax}S/;Zdk vuqikr ifjdfyr dhft, ;fn budh xfrt Å tkZdk
vuqikr 1 : 18 gksa&
(A) 1:23 (B*) 3 : 2 (C) 2 : 3 (D) 23:1](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-23-320.jpg)




![Page #
11
(C) Both are chain isomers
(D*) Both have same general formula.
rFkk
mijksDr nkslajpukvksadsfy, dkSulk@dkSulsdFku lR; gS%
(A*) izR;sd esagkbMªkstu U;wurkdklwpdkad rhu gSA
(B*) nksuksae/;ko;ohgSaA
(C) nksuksaJ`a[kykleko;ohgSaA
(D*) nksuksaleku lkekU; lw=kj[krsgSaA
Integer(Double)(3)
56. An LED of powers X watt emits twice as many photons at 1000 nm as another LED of power 5
watt at 400 nm in one second. Find X.
(ATS(P))
1 lSd.M esaX okWV 'kfDr dh,d LED }kjkmRlftZr 1000 nm dsQksVksuksadhla[;k]5 okWV 'kfDr dhvU;
LED }kjkmRlftZr 400 nm dsQksVksuksadhla[;kdhrqyukesanqxquhgksrhgS]rc X Kkr dhft,A
Ans. 04
Sol. Time = 1 second LED 1 ; 15
2LED
Energy = X × 1 = XJ ; 5J
= 1000 nm ; = 400 nm
no. of photons = 2n ; no. of photon = n
1000
hc
n2X ;
400
hc
n5
1000
54002
X
= 4.
Sol. le; = 1 lSd.M LED 1 ; 15
2LED
Å tkZ= X × 1 = XJ ; 5J
= 1000 nm ; = 400 nm
QksVkWuksadhla[;k= 2n ; QksVkWuksadhla[;k= n
1000
hc
n2X ;
400
hc
n5
1000
54002
X
= 4.
57. Threshold frequency of a metal is 0. When light of frequency = 30 is incident on the metal
plate, maximum kinetic energy of emitted photoelectron is x. When frequency of incident radiation
is 50, kinetic energy of emitted photoelectron is y. If threshold energy of metal is z. Find value of
2
z
yx
: (ATS(P))](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-30-320.jpg)
![Page #
12
,d /kkrqdh nsgyhvko`fÙk0 gSA tc /kkrqIysV ij vko`fÙk = 30 dk izdk'k vkifrr gksrk gS]rksmRlftZr
izdk'k]bySDVªkWu dh vf/kdre xfrt Å tkZx gSA tc vkifrr fofdj.k dh vko`fÙk 50 gS]rksmRlftZr izdk'k
bySDVªkWu dhxfrt Å tkZy gSA ;fn /kkrqdhnsgyhÅ tkZz gS]rks
2
z
yx
dkeku Kkr dhft,A
Ans. 36
Sol. 0 0 0x 3h h 2h
0 0 0y 5h h 4h
0z h
2
x y
z
= 36.
58. An element exist in three isotopic form : 60A, 62A and 64A.
Relative abundance of 60A = 30% by mole.
If average atomic mass of ‘A' is 62.6 u, find out the sum of % abundance (by mole)
of 60A and 64A. (Mol-1)(P))
;fn ,d rRo]rhu leLFkkfud :i 60A, 62A rFkk64A esaik;ktkrkgSA
60A dhvkisf{kd ckgqY;rk= 30%
;fn ‘A' dk vkSlr ijek.oh; nzO;eku 62.6 u gS]rks60A o 64A dh izfr'kr ckgqY;rk ¼eksy@eksy esa½ dk
;ksx Kkr dhft,&
Ans. 90
Sol. Average atomic mass =
(30 60) (70 x)62 (x 64)
100
x = 60%
mole% of
60
A + mole% of
64
A = 90
Sol. vkSlr ijek.oh; nzO;eku =
(30 60) (70 x)62 (x 64)
100
x = 60%
60
A dkeksy % +
64
A dkeksy % = 90
Integer(Double)(2)
59. The sum of number of functional group and index of hydrogen deficiency in the following
compound is -
fuEu ;kSfxd easfØ;kRed lewg o gkbMªkstu U;wurkdklwpdkad dhla[;kdk;ksx gSA
(IUPAC(O))
OH
O
N
NH2 COOH
Ans. 19
Sol. Index of hydrogen deficiency = 15
Functional group = 4
gkbMªkstu U;wurkdklwpdkad = 15
fØ;kRed lewg = 4
60. How many isomers are possible of molecular formula C7H16 having word root pent.
(Isomerism(O))
C7H16 v.kqlw=kj[kusokys;kSfxd ftldkewy 'kCn isUV gS]dsfdrusleko;ohlEHko gS
Ans. 05](https://image.slidesharecdn.com/1-150420004801-conversion-gate02/85/1-ct-1-paper-1-10-aug-2014-31-320.jpg)
