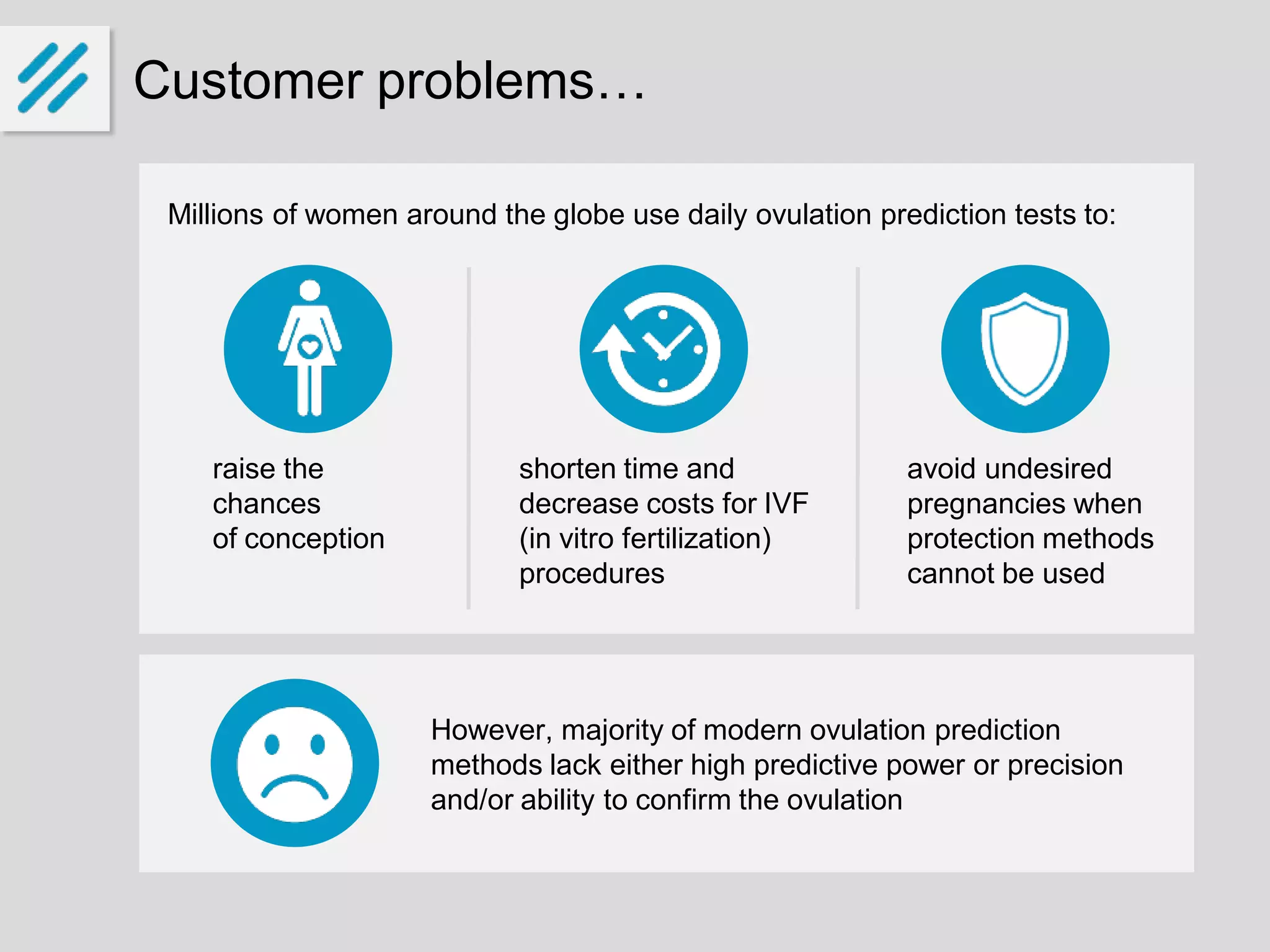
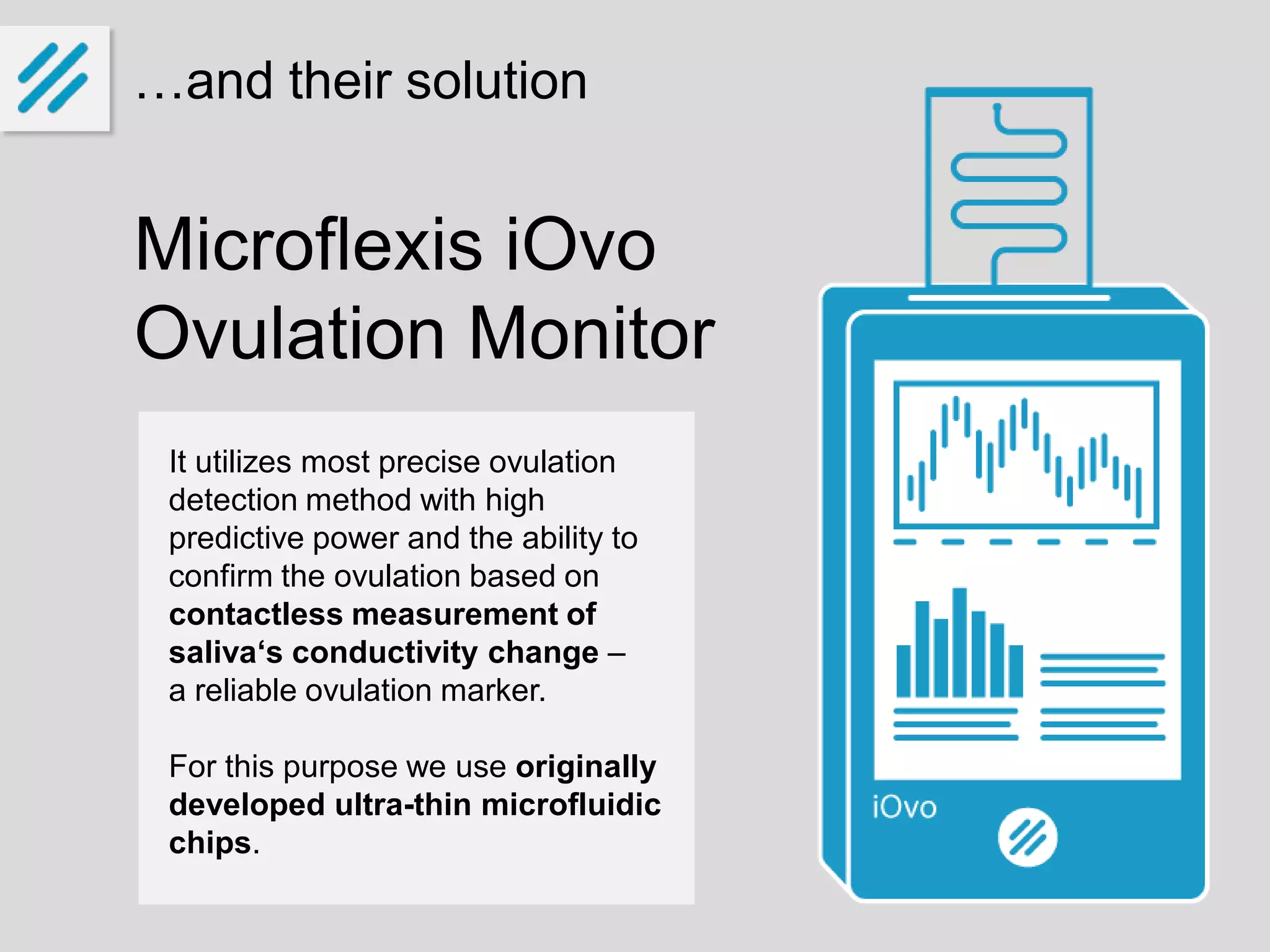
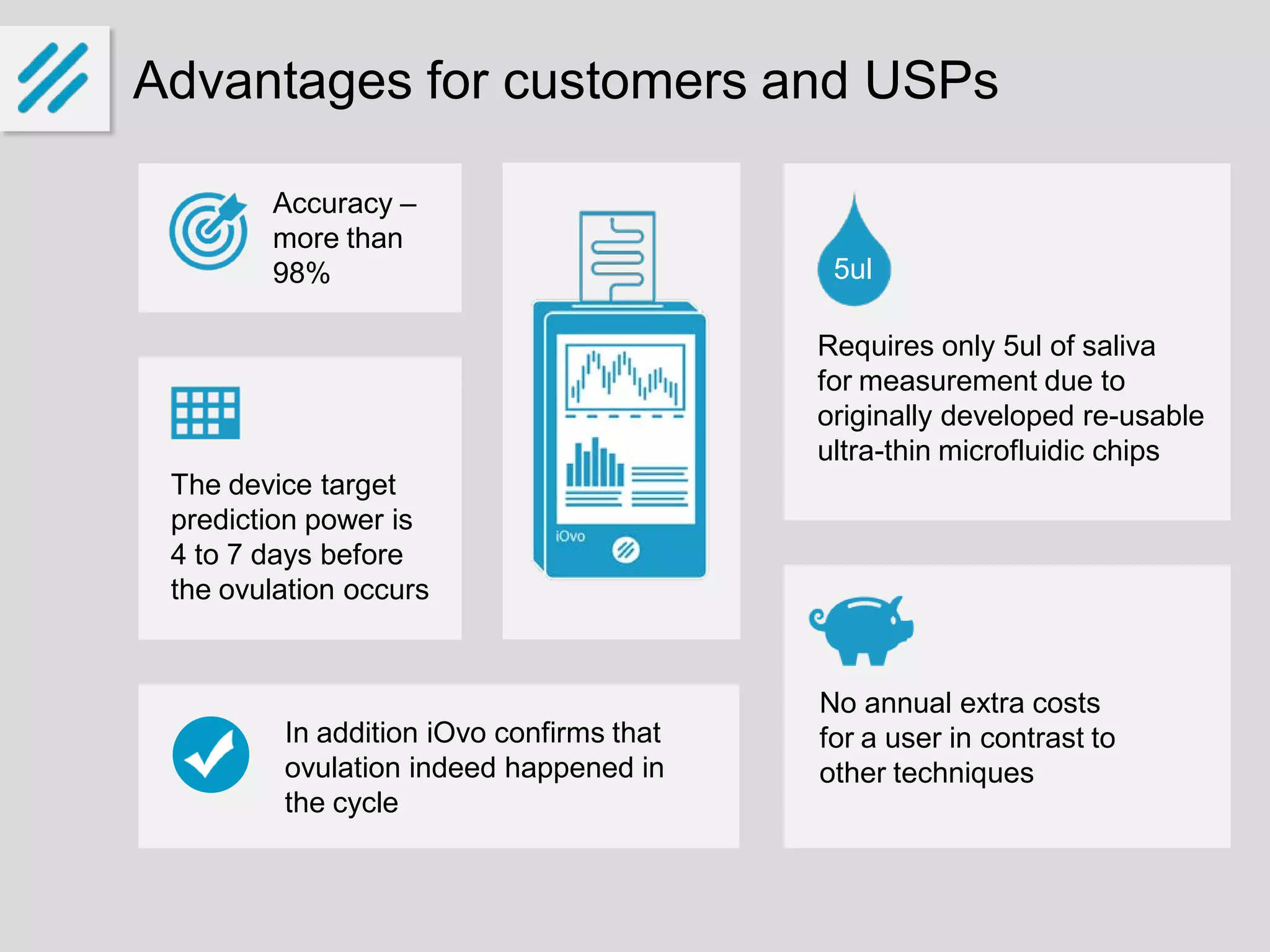
The document describes a new ovulation monitor called the iOvo that uses microfluidic chips to precisely detect ovulation through measuring changes in saliva conductivity, a reliable marker of ovulation. It aims to provide more accurate predictions of ovulation 4-7 days in advance compared to other methods, and can confirm ovulation occurred. The founders have invested their own money and are seeking additional funding of around 10 million euros over 5 years for development, testing, patenting and production ramp-up. They expect to sell over 125,000 units in 5 years, reaching break-even in the third year and generating a 28% return on investment. The global market for fertility and ovulation products is growing and valued at around $4 billion annually.