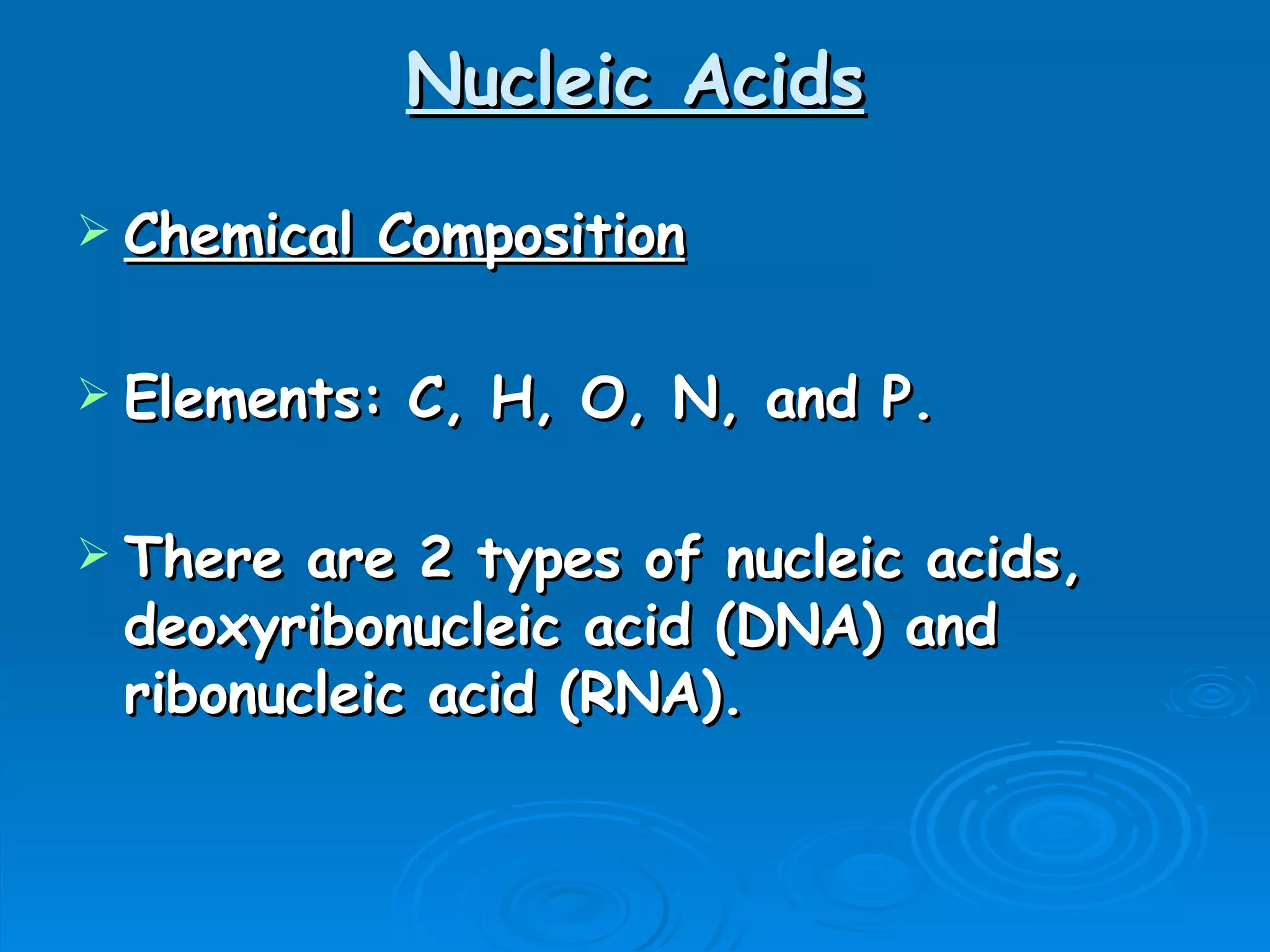
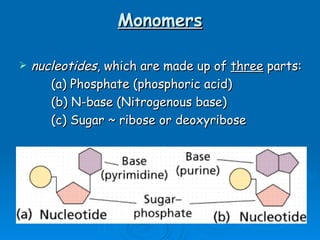
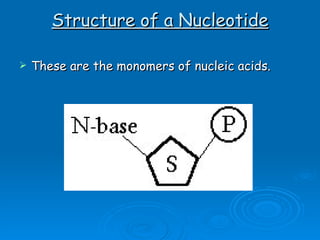
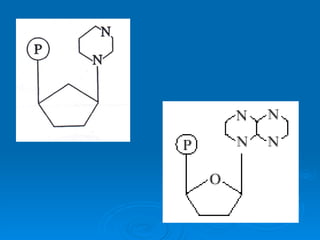
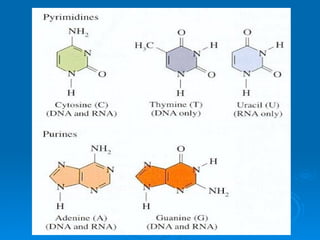
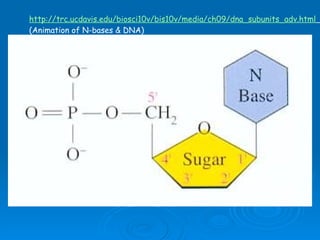
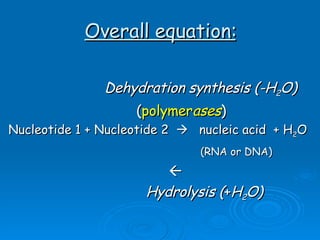
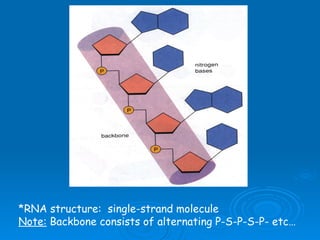
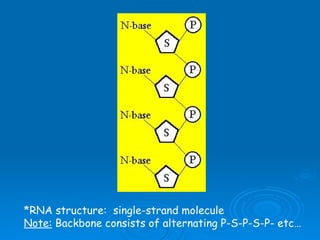
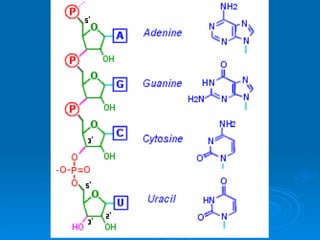
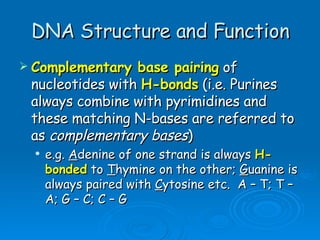
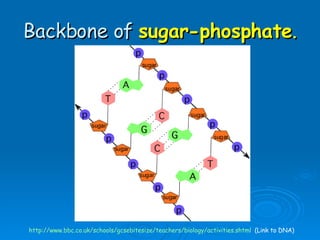
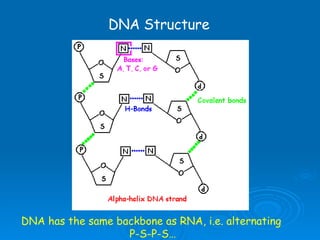
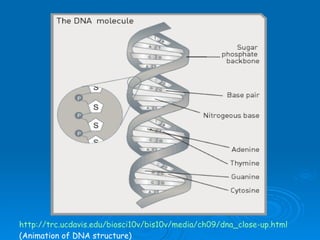
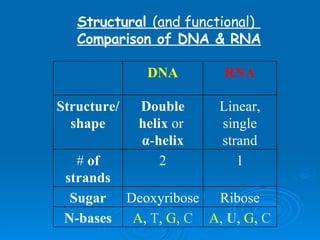
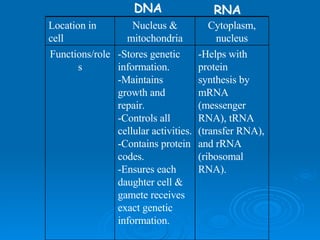
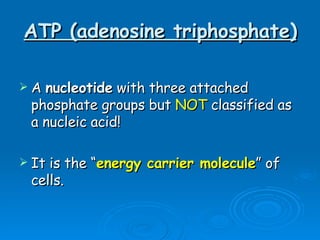
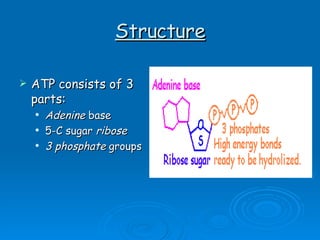
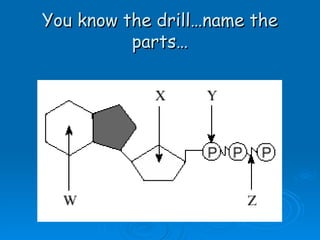
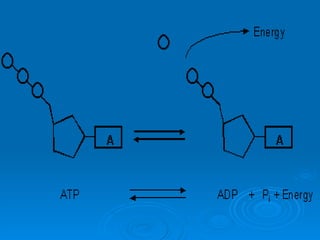
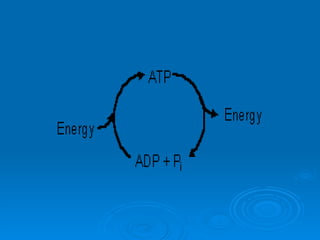
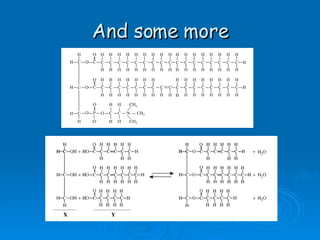
The document discusses the chemical composition and structure of nucleic acids. There are two main types of nucleic acids - DNA and RNA. Both are composed of nucleotides, which contain a phosphate group, a sugar (ribose in RNA, deoxyribose in DNA), and a nitrogenous base. DNA forms a double helix structure in the nucleus and stores genetic information. RNA is single-stranded and found in the nucleus and cytoplasm, assisting with protein synthesis through messenger RNA, transfer RNA and ribosomal RNA.