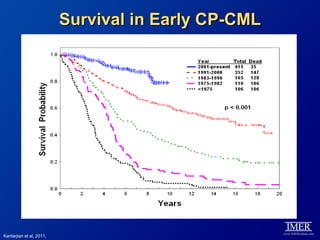
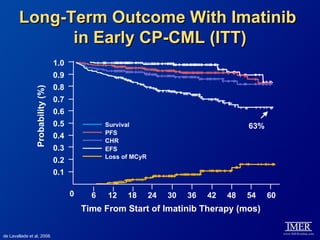
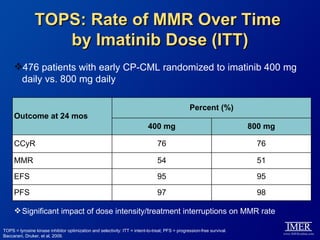
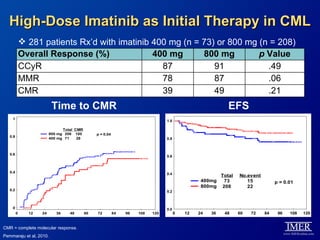
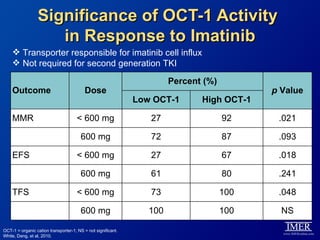
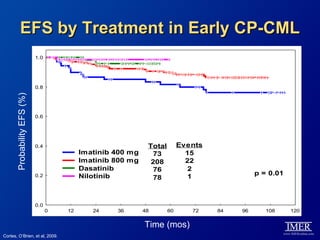
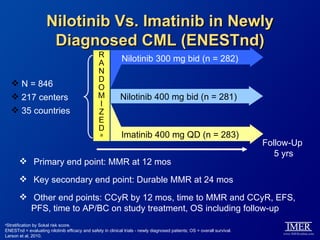
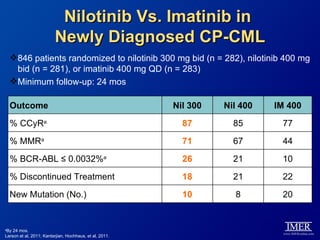
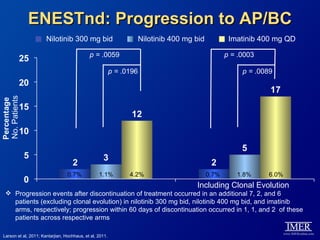
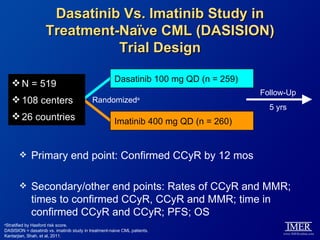
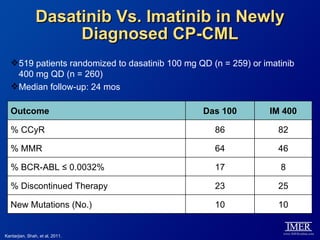
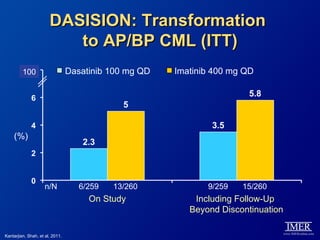
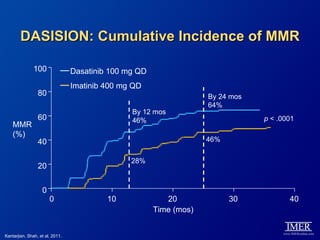
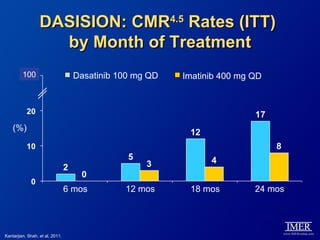
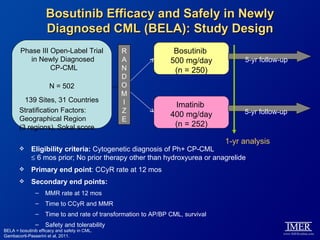
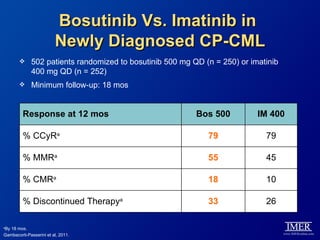
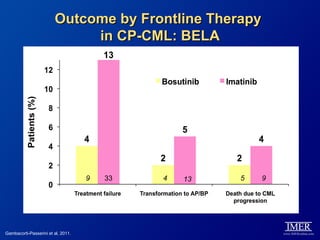
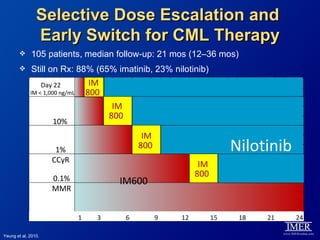
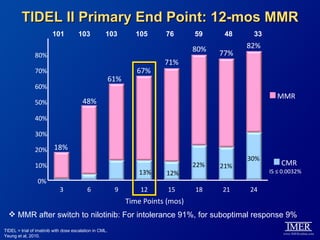
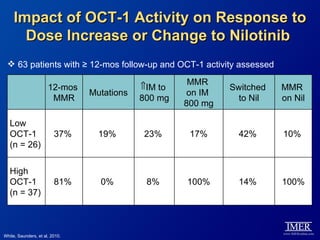
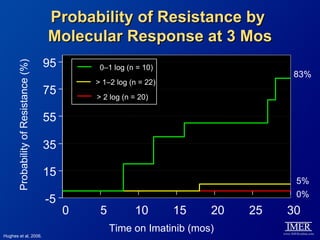
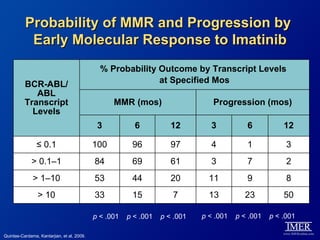
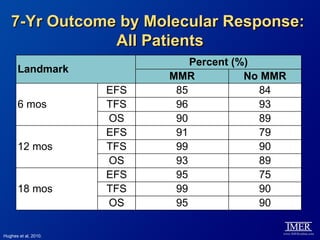
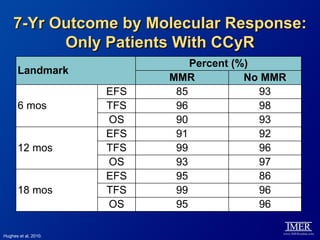
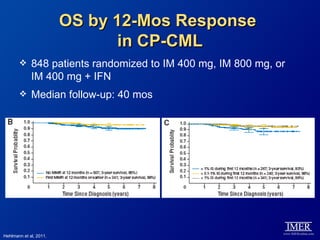
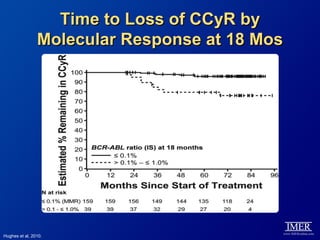
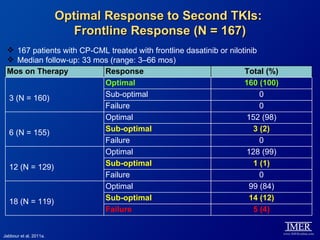
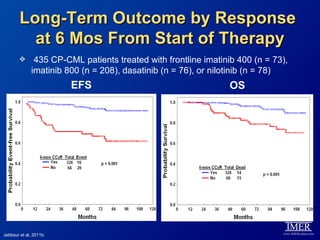
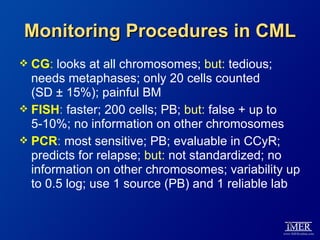
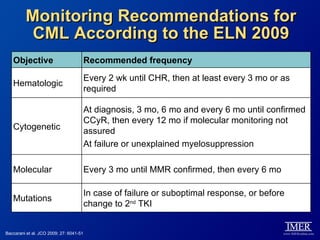
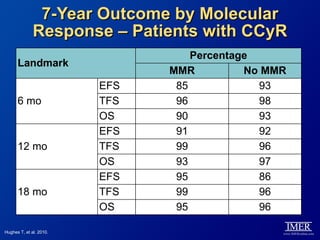
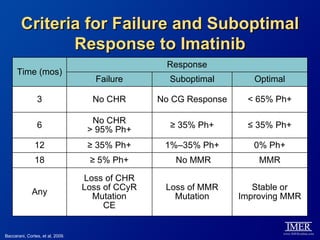
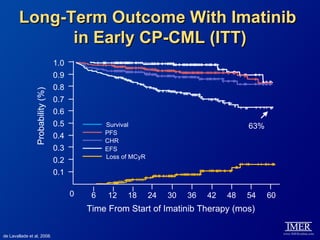
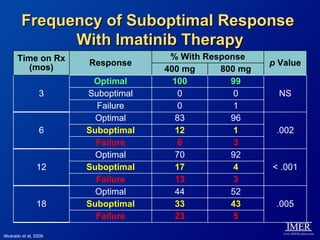
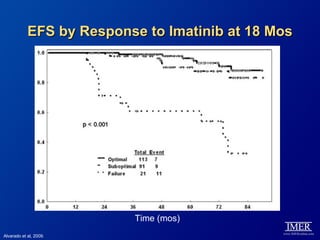
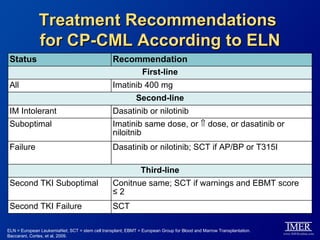
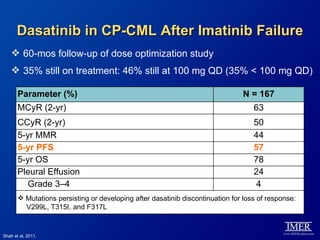
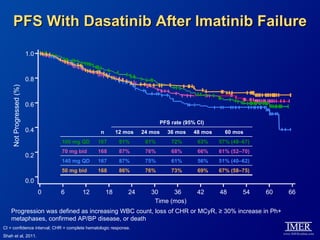
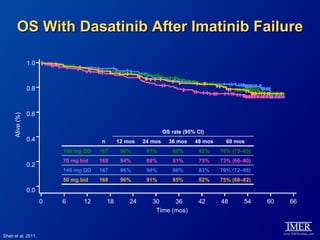
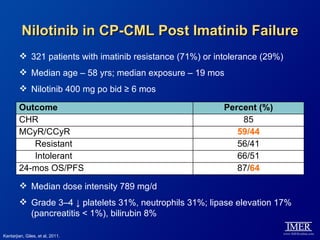
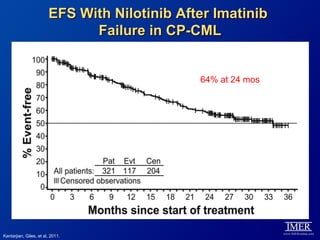
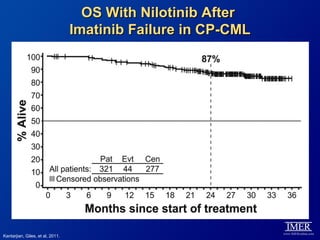
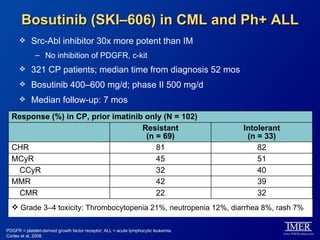
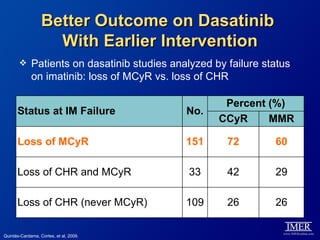
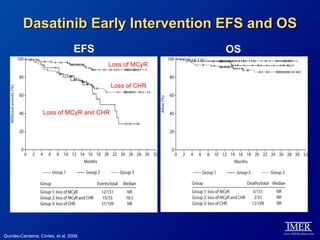
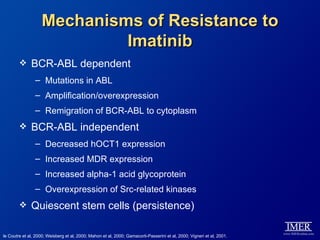
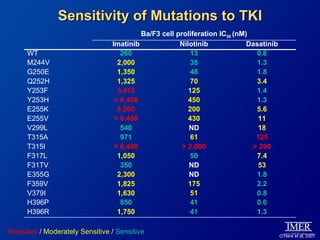
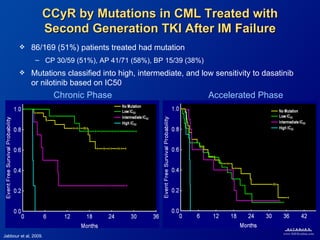
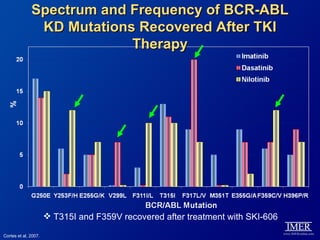
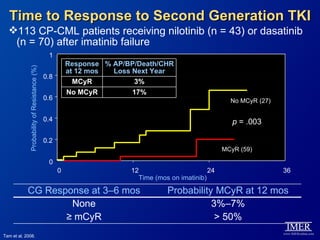
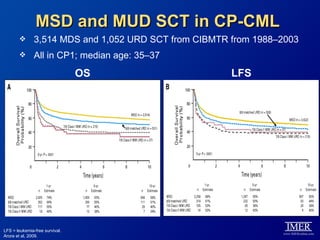
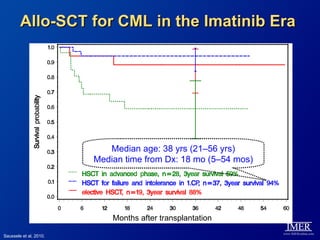
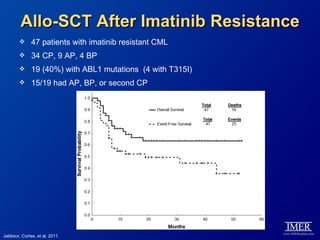
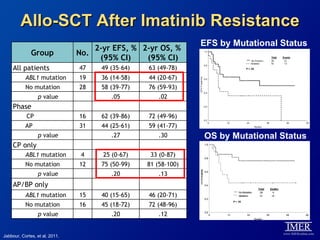
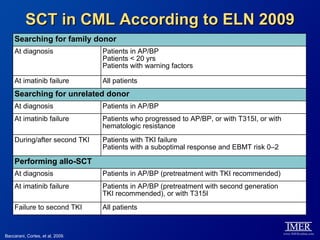
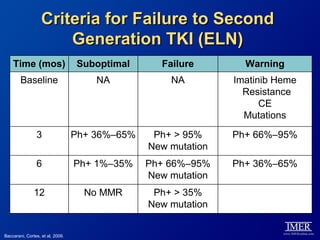
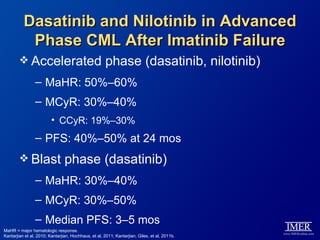
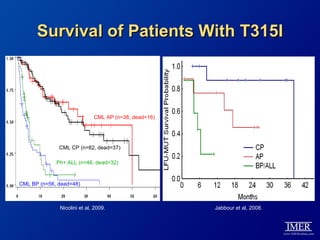
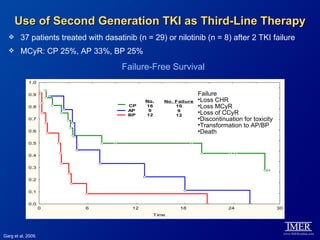
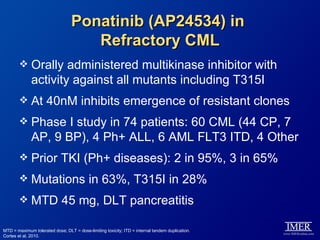
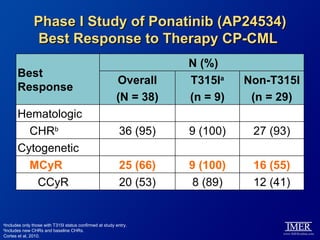
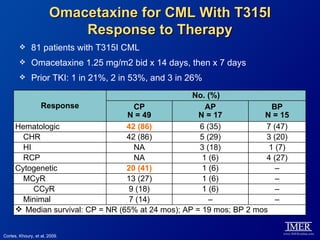
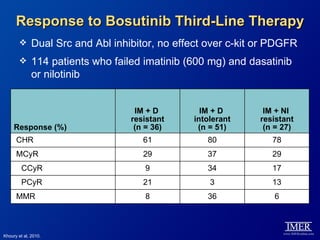
This slide deck, intended for educational purposes, provides information about chronic myeloid leukemia (CML) therapies, diagnostic approaches, and management strategies, reflecting views of various medical faculty. Key learning objectives include optimal therapy selection based on patient characteristics, understanding laboratory tests for diagnosis and monitoring, and addressing relapses and second-line treatment options. Disclaimers emphasize that the content does not substitute professional medical advice and may discuss unapproved drug uses.