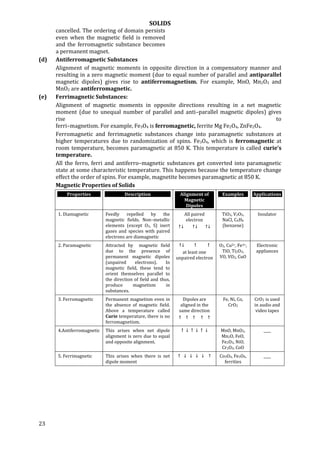
This document discusses different types of solids and their properties. It describes amorphous solids as having atoms arranged irregularly without a characteristic shape, and crystalline solids as having atoms arranged regularly in defined planes. Amorphous solids are isotropic while crystalline solids are anisotropic. Crystalline solids have sharp melting points and cleavage planes, while amorphous solids soften over a range of temperatures and have irregular surfaces when cut. The document also discusses the unit cell and space lattice of crystalline solids.








![SOLIDS
13
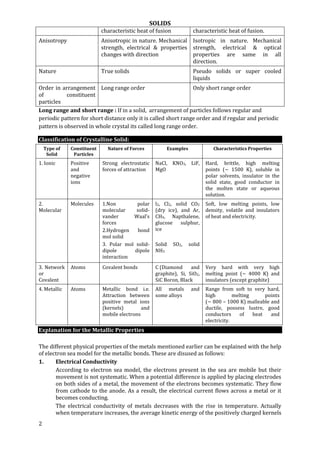
Illustrating effect of radius ratio on co–ordination number. As rc/ra increases, coordination
number increases
Q. 1 Atoms of element B from hcp lattice and those of the element A occupy 2/3rd of tetrahedral
voids. What is the formula of the compound formed by these elements A and B?
Q.2 In a crystalline solid, anions B are arranged in a cubic close packing. Cations A are equally
distributed between octahedral and tetrahedral voids. If all the octahedral voids are
occupied, what is the formula of the solid?
Q.3 In the mineral, spinel, having the formula MgAl2O4, oxide ions are arranged in the cubic close
packing, Mg2+ ions occupy the tetrahedral voids while Al3+ ions occupy the octahedral
voids.
(i) What percentage of tetrahedral voids is occupied by Mg2+ ions?
(ii) What percentage of octahedral voids is occupied by Al3+ ions?
Q.4 In a solid oxide ions are arranged in cubic close packing. One-sixth of the tetrahedral
voids are occupied by cations A while one-third of the octahedral void are occupied by
the cations B. What is the formula of the solid?
Q.5 B– ions form a close packed structure. If the radius of B– ion is 195 pm, calculate the
radius of the cation that just fits into the tetrahedral hole. Can a cation A+ having a radius
of 82 pm be slipped into the octahedral hole of the crystal A+ B–?
Q.6 If the close packed cations in an NaCl type solid have a radius of 75 pm, what would be
the maximum and minimum sizes of the anions forming the voids?
Density of Cubic Unit Cell
In NaCl type unit cell, d = rc + ra = a/2
In CsCl type d = rc + ra = a
2
3
[2d = Body diagonal]
In ZnS type 4ra = a2
Q.1 CsCl has cubic structure. Its density is 4.0g cm–3. What is the distance between Cs+ and
Cl– ions? (At. mass of Cs = 133) 357 pm](https://image.slidesharecdn.com/solid-171009133702/85/Solid-CLASS-XII-13-320.jpg)
![SOLIDS
15
the electrons. Free electrons and holes in crystals are considered to be electronic
imperfections. These electronic imperfections are responsible for electrical
conductance.
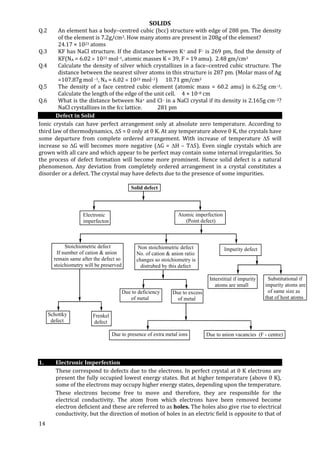
2. Point Defect
If atoms are missing from lattice point, displaced or extra ions are present, it is called
point defects [electrical neutrality of the crystal is never affected by any kind of defect].
There are three types of point defects
(a) Stoichiometric defect (b) Non–stoichiometric (c) Impurity defects
(A) Stoichiometric defect:
In which stoichiometry of crystal is maintained even after the defect. i.e. Number of
positive & negative ions are exactly in the same ratios as in non–defected solids.
(i) Schottky defect
It is basically a vacancy defect in ionic solid. When a crystal site become vacant by
removal of a structural unit in the lattice, then the defect is referred to as the vacancy
defect. In an ionic crystal, a cation and anion may leave the lattice to cause two vacancies.
Such a defect which involves equal no of cation and anion vacancies in the crystal lattice
is called a schottky defect. Cation vacancy is followed by anion vacancy leading to
decrease in density.
Compound with high Co. no. & high ionic character shows this defect. The crystal
begins to conduct electricity to a smaller extent by ionic mechanism. The stability of the
crystal is lowered. e.g. NaCl, CsCl. It has been observed that in NaCl, there are about 106
Schottky pair per cm3. In 1 cm3 there are about 1022 ions. Thus there is one schottky
defect per 1016 ion.
(ii) Frenkel Defect
This defect is shown by ionic solids. The small ion (usually cation) is dislocated from its
normal site to an interstitial site. It creates a vacancy defect at its original site & an
interstitial defect at its new location. Small ions are displaced from their standard lattice
point and present in non–standard lattice points i.e. interstitial. This is also called
dislocation defect. It does not change the density of the solid as atoms are not absent
from lattice they are just displaced. This defect is shown by those ionic solids in which
there is a large difference in size of ions and low co.od(less ionic character] e.g. Zng,
AgCl, Agl, AgBr due to presence of small Zn+2 and Ag+ .
AgBr shows both Schottky and Frenkel defect. (It is because AgBr crystal has a
coordination number of 6 which is neither high nor low. The radius ratio of AgBr is also
intermediate, thus AgBr satisfies the conditions to exhibit both type of defects.)
Consequences of Schottky and Frenkel Defect
Schottky & Frenkel defects in crystals lead to some interesting consequences. These are given
below:
1. Because of the presence of these defects, the electrical conductivity of crystal increases.
When an electric field is applied, a nearby ion moves from its lattice site to occupy a
‘hole’. This results in creating a new ‘hole’ and another nearby ion moves into it and so
on. This process continues and hole, thereby, move from one end to the another end.
Thus, it conducts electricity across the whole of the crystal.](https://image.slidesharecdn.com/solid-171009133702/85/Solid-CLASS-XII-15-320.jpg)