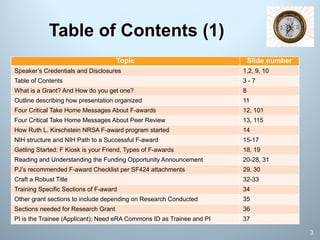
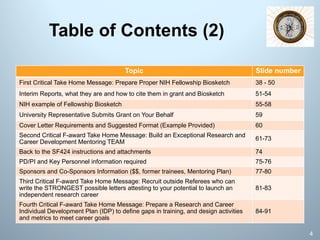
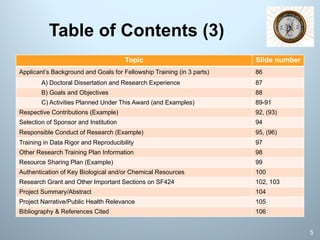
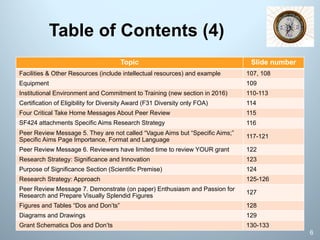
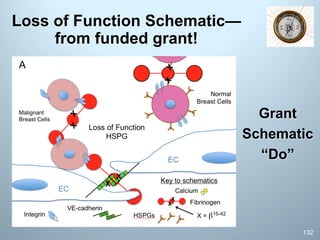
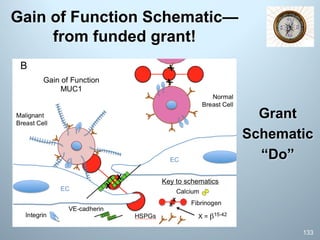
This document is a comprehensive guide on preparing NIH F-award applications, providing essential principles and a structured approach to grant writing. It emphasizes the need for a proper biosketch, an exceptional mentoring team, strong recommendation letters, and an individualized development plan. The presentation details sections of the grant, critical take-home messages about peer review, and resources for successfully navigating the NIH grant process.









































![How to Cite Interim
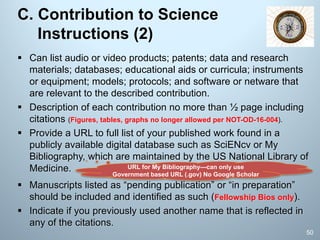
Research Products
• To cite the product, must include the Digital Object
Identifier (doi) and the Object type (e.g. preprint,
protocol) in the citation.
• List any information about the document version (e.g.
most recent date modified), and if relevant, the date
the product was cited.
– Example: Bar DZ, Atkatsh K, Tavarez U, Erdos MR, Gruenbaum Y, Collins FS.
Biotinylation by antibody recognition- A novel method for proximity labeling.
BioRxiv 069187 [Preprint]. August 11, 2016 [cited 2017 Jan 12]. Available
from: https://doi.org/10.1101/069187.
• Proper citing of preprints helps reviewers understand that the
product is public, interim, and identifies the specific version that
is being referenced.
54](https://image.slidesharecdn.com/tl1f-awardapplicationworkshopupdate17april2015-150412232343-conversion-gate01/85/TL1-NRSA-F-award-application-workshop-and-How-to-Prepare-Complete-Application-54-320.jpg)


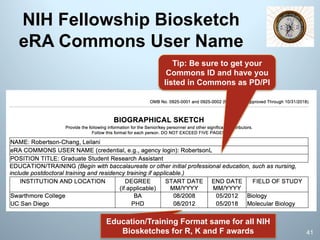
![§ STICK to one format of your name for publication.
§ If you have a middle name use initial, especially if your
name is common and there are dozens of you in
PubMed!
§ Don’t do what I did—Published under 3 versions of name:
v PJ Simpson, PJ Haidaris, and PJ Simpson-Haidaris—eek
§ On my Biosketch, I used to include:
The following search string will retrieve the PI’s citations in PubMed:
v ("simpson-haidaris pj"[AU] OR "haidaris pj"[AU] OR “haidaris p” OR "simpson
pj"[AU] AND Rochester[AD]) OR ("simpson pj"[AU] AND "Gene"[Journal]) OR
("simpson-haidaris pj"[AU] AND "Thromb Res"[Journal]) OR ("simpson-haidaris
pj"[AU] AND "J Thromb Haemost"[Journal]) OR (“haidaris p”[AU] AND “Thromb
Haemost”[Journal])
§ Now I include:
v My NCBI | My Bibliography: http://www.ncbi.nlm.nih.gov/sites/myncbi/pj.simpson-
haidaris.1/bibliography/40100400/public/?sort=date&direction=ascending
NIH Biosketch Pointer
57](https://image.slidesharecdn.com/tl1f-awardapplicationworkshopupdate17april2015-150412232343-conversion-gate01/85/TL1-NRSA-F-award-application-workshop-and-How-to-Prepare-Complete-Application-57-320.jpg)













































































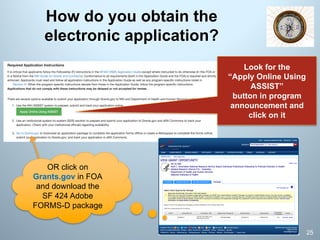
![FOA-specific I Instructions and
Found in Section IV!
Required Application Instructions
It is critical that applicants follow the Fellowship (F) instructions in the
SF424 (R&R) Application Guide [URL below] EXCEPT where instructed
to do otherwise (this FOA or a Notice [NOT] from the NIH Guide for
Grants and Contracts). Conformance to all requirements (both in the
Application Guide and the FOA) is required and strictly enforced.
Applicants must read and follow all application instructions in the
Application Guide as well as any program-specific instructions noted in
Section IV [of F-award parent FOA]. When the program-specific
instructions [i.e., NRSA F-fellowship program] deviate from those in the
Application Guide, follow the program-specific [in FOA] instructions.
Applications that do not comply with these instructions may be
delayed or NOT accepted for review.
Verbatim instructions from PA-16-308
https://grants.nih.gov/grants/how-to-apply-application-guide/forms-d/fellowship-forms-d.pdf
145](https://image.slidesharecdn.com/tl1f-awardapplicationworkshopupdate17april2015-150412232343-conversion-gate01/85/TL1-NRSA-F-award-application-workshop-and-How-to-Prepare-Complete-Application-145-320.jpg)
![What does it take to write an
F-award application?
(refusal to take “No” for an answer) [1]
• Allow plenty of time to complete all sections.
– make sure when editing sections, such as goals you
also edit activities planned to accommodate changes in
goals.
– same goes for specific aims and research strategy—if
you add or delete an aim, make sure the experimental
design matches!
• Recommend you have at least 1 first-author
publication for F31/F30 (at least submitted) and
two-three for F32.
146](https://image.slidesharecdn.com/tl1f-awardapplicationworkshopupdate17april2015-150412232343-conversion-gate01/85/TL1-NRSA-F-award-application-workshop-and-How-to-Prepare-Complete-Application-146-320.jpg)
![What does it take to write an
F-award application?
(refusal to take “No” for an answer) [2]
• Enlist mentors and outside referees early and
discuss project and goals so they can commit to
participate and write supportive letters because
they KNOW you.
• Work with sponsor and university representative
to complete all aspects of project.
• Read ALL instructions and Pay Attention to detail;
let others read, & edit, edit, edit.
• Oh, and edit some more after putting it aside for
awhile. 147](https://image.slidesharecdn.com/tl1f-awardapplicationworkshopupdate17april2015-150412232343-conversion-gate01/85/TL1-NRSA-F-award-application-workshop-and-How-to-Prepare-Complete-Application-147-320.jpg)





