Embed presentation
Downloaded 41 times

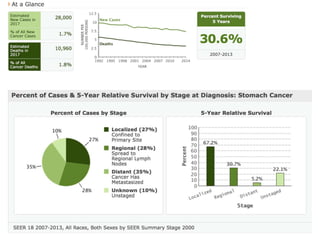
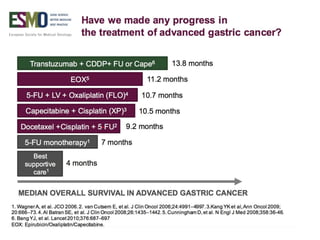
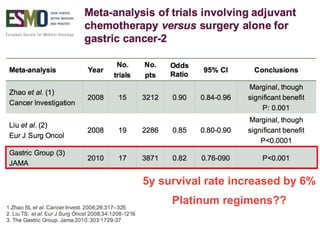


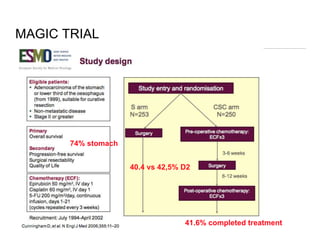
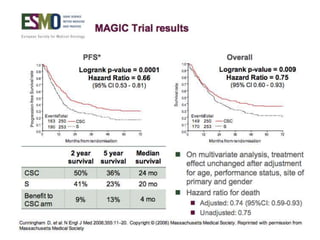
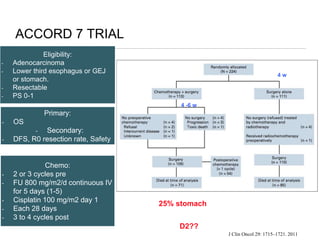
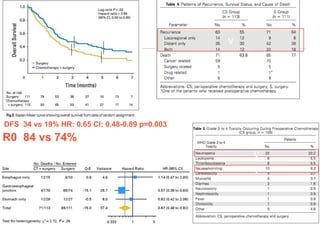
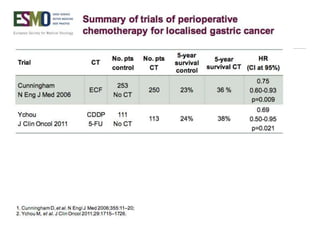
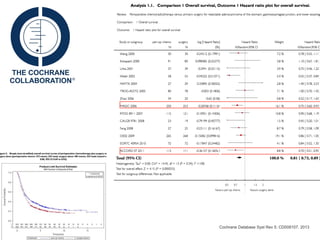
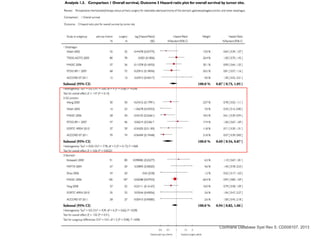
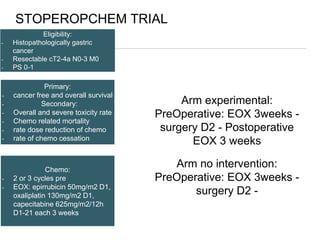
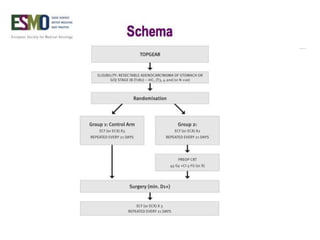
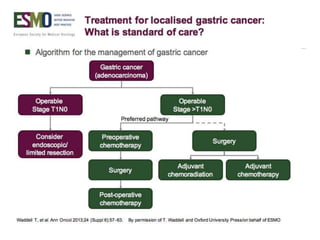
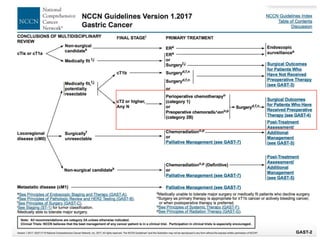


This document discusses several studies on neoadjuvant chemotherapy for gastric cancer. It summarizes the MAGIC trial which found that platinum-based neoadjuvant chemotherapy improved 5-year survival by 6% compared to surgery alone. It also discusses the ACCORD 07 trial which found that neoadjuvant chemotherapy led to higher R0 resection rates and improved disease-free survival compared to surgery alone. Finally, it summarizes the STOPEROPCHEM trial comparing neoadjuvant chemotherapy followed by surgery to surgery alone for resectable gastric cancer. The document concludes that a multidisciplinary team approach is positive and perioperative chemotherapy can induce downstaging, increase R0 resection rates, and improve disease-free and overall survival

















