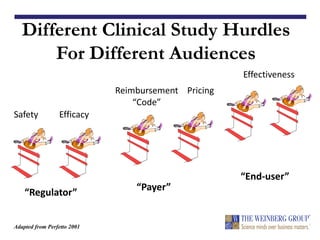
The document discusses the design and regulation of clinical studies for medical devices, highlighting key approval standards such as PMA and 510(k). It emphasizes the importance of substantial equivalence, the types of clinical trials required, and the role of evidence in determining safety and effectiveness. Practical considerations for trial execution, data analysis, and regulatory compliance are also outlined, alongside the need for a well-structured trial protocol.