Power Notes Atomic Structure 2014c
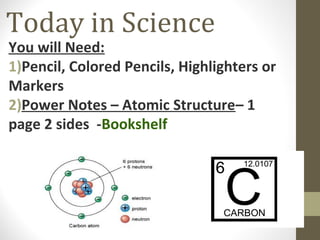
- 1. Today in Science You will Need: 1)Pencil, Colored Pencils, Highlighters or Markers 2)Power Notes – Atomic Structure– 1 page 2 sides -Bookshelf
- 2. Final Jeopardy You will Need: 1)Pencil
- 3. FINAL JEOPARDY • ANSWER: Complete the Chart Item Helium H2O at 32 degrees C Pencil The element Mercury Has Mass Definite Volume Definite Shape Takes the shape of container What is it at room temp: Solid, Liquid, Gas, Plasma
- 4. FINAL JEOPARDY • ANSWER: Complete the Chart Item Has Mass Definite Volume Helium X H2O at 32 degrees C X X Pencil X X The element Mercury X X Definite Shape Takes the shape of container At room temp: Solid, Liquid, Gas, Plasma X Gas X Liquid X Solid X Liquid
- 5. 1.MANAGER– Log in to W e LearningPoint – are Lead discussion Cha ngin Sea t s ! ! gOrganize 2. MATERIALS MANAGER–! !! Table Box 3. TIMER/DESIGNER– “Highlighting Patrol” 4.ORAL PRESENTER– Communicator, Asks Questions for the group
- 6. 1.MANAGER– Log in to LearningPoint – Lead discussion 2. MATERIALS MANAGER– Organize Table Box (Colored Pencils, Markers, 4 rulers, glue sticks, tape, scissors, post-it notes) 3. TIMER/DESIGNER– “Highlighting Patrol” 4.ORAL PRESENTER– Communicator, Asks Questions for the group
- 7. Topic: Power Notes- Atomic Structure Do we understand everything????
- 8. Democritus 400 BC Greece - stated that all matter is made up of atoms. He also stated that atoms are eternal and invisible and so small that they can’t be divided, and they entirely fill up the space they’re in Lavoisier 1789 France - provided the formula for the conservation of matter in chemical reactions, and also distinguished between an element and a compound • 1st idea of “atoms” • Believed all matter is made up of tiny particles separated by space • Named particles “atomos” which means indivisible. • Founder of modern chemistry • 1st carefully controlled experiments providing evidence to the Law of Conservation of Mass
- 9. Dalton 1766-1844 England - formed the 1st atomic theory, which states that all matter is composed of tiny, indestructible particles called atoms that are all alike and have the same atomic weight. 1st MODERN Atomic Theory 1.All matter made up of tiny indivisible particles called atoms 2.Atoms of the same element have identical properties 3.Atoms of different elements have different properties 4.Atoms combine in a specific ratio to form compounds 5.A specific compound is always made up of atoms in a specific proportion.
- 10. Thomson 1897 Plum Pudding Model 1.Negative particles scrambled into the “dough” of the positive particles. England - discovered the electron and developed the plumpudding model of the atom. Rutherford 1898 England - used the results of his gold-foil experiment to state that all the mass of an atom was in a small positively-charged ball at the center of the atom. Gold Foil Experiment 1.Atoms are mostly empty space 2.All the Mass of an atom is in the positively charged ball in the center of the structure.
- 11. •Scientists look for patterns in data and passed experiments to What is the difference between the EXPECTED PREDICT what they think might RESULTS &OBSERVED happen. •When scientists actually do an RESULTS? experiment their observed results may be different than what they EXPECT to happen because of passed information. •However, many times the expected results are in fact, what is observed.
- 12. Bohr 1922 Denmark - stated that the electrons moved around the nucleus in successively large orbits. He also presented the Bohr atomic model which stated that atoms absorb or emit radiation only when the electrons abruptly jump between allowed, or stationary, states. Schrödinger (Schroedinger ) Austria - introduced the 1930 Shroedinger Equation, a wave equation that describes the form of the probability waves that govern the motion of small particles and how these waves are altered by external influences. “Planetary” atom model 1.Believed the atom structure was like our solar system 2. Nucleus in the middle like the sun and the electrons orbiting like the planets. Wave Mechanic Model 1.Planetary model was too specific 2.Rather: electrons vibrate around the outside of the nucleus- can only predict where they are most like to be 3.QUARKS – protons, neutrons and electrons made up of some of these smaller particles. 4.http://library.thinkquest.org/05aug/01087/quarks.html
- 13. Basic Atomic Structure Today scientists agree on the three basic subatomic particles that make up all atoms. Bohr Model for Lithium Electron = 3 (not to scale) Make sure to include these question next to your model P= 3 + N = 3 0 Answer s Where is most of the mass in the Atom? NUCLEUS Where is most of the volume in the Atom? Outside the Nucleus; Electron Cloud
- 14. Nucleus • • • No need to include words… just illustration Electron • • • • Contains protons(+) and Neutrons (0) Holds most of the mass of the atom Very small compared to the entire size of the atom Most dense part of the atom Negatively charged subatomic particle Found outside the nucleus in the electron cloud Smallest particle; mass = 1/1836th of the atom
- 15. Proton • • • • Neutron • • • • • • Positively charged subatomic particle, Found in the nucleus Mass = 1 amu (atomic mass unit) # of protons in an atom IDENTIFIES the atom (which element) # of protons in the nucleus of an atom called the atomic number Zero charge Mass = 1 amu Do NOT affect the identity of the atom Found in the nucleus Same atom of an element can have varying amounts of neutrons (isotope) Adding the total number of protons & neutrons = Atomic Mass Number http://www.sawyerscience.com/Units/unit2/atoms_compounds.html
- 16. 1. Atomic Mass on the Periodic Table represents a “weighted average” of the mass of all the naturally occurring ISOTOPES of each element. (based on mass & abundance of each isotope.) Atomic Number 1. Number of PROTONS (Unique to each element) 2. Number of ELECTRONS (IF Atom is NEUTRAL) 2. Chemical Symbol 1. Abbreviation of element (some from LATIN name) 3. Element Name 1. Full name provided below symbol 4. Atomic Mass Number 1. Sum of the particles in the nucleus 2. Represents total count of protons and neutrons 3. Positively charged because neutrons have no charge and protons are positive
- 17. All atoms found on the • Periodic Table are NEUTRAL– same # of protons(+) and # of electrons(-) Isotope Atoms of the same element with same number of protons and different number of neutrons. • Hydrogen has 3 natural occurring ISOTOPES: Protium, Deuterium and Tritium are All ROGEN have l HYD use al a bec oton. r 1 p All ISOTOPES of Hydrogen are neutral because they have 1 electron as well. Make sure to include the electron in your illustration. (Did you put the electron in the right place?)
- 18. IONS • • When atoms gain or lose electrons they become charged. (number of protons (+) and electrons (-) particles are NOT EQUAL) Atoms that do not have equal amounts of protons (+) and electrons (-) particles are called IONS Draw atoms of hydrogen that are charged because of the UNEQUAL amounts of protons and electrons. Review REVIEW & REFLECT Short answer, no complete sentences. All answers are located in your notes. + - On ALL pages – Please do this with a colored pen, pencil or marker.
- 19. Homework 1)None Unless you owe me something (We will continue working on Monday and Tuesday of next week) 2)Have a good weekend! Do You see HOW the periodic table and the Atomic Structure are RELATED?