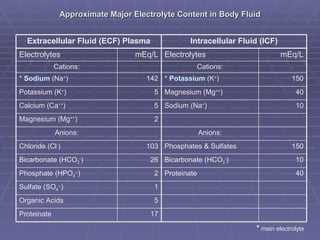
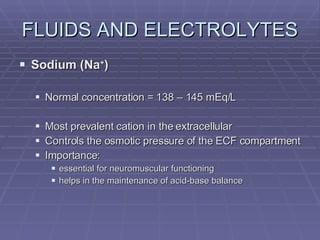
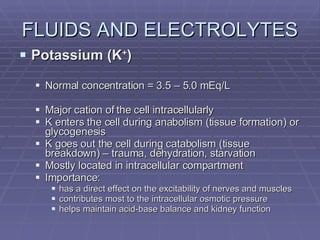
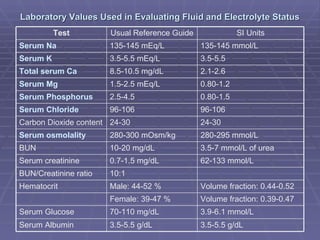
Dr. Ronald Sanchez Magbitang is an esteemed physician with a B.S. in Biology and a Doctor of Medicine from reputable institutions, currently serving as the Chief of Hospital at Gov. Eduardo L. Joson Memorial Hospital. His extensive training includes a focus on internal medicine, hematology, and HIV/AIDS, alongside multiple leadership roles in various medical associations in the Philippines. The document elaborates on the essential concepts of body fluids and electrolytes, their functions, compartments, balance, and the physiological mechanisms involved in maintaining fluid and electrolyte homeostasis.















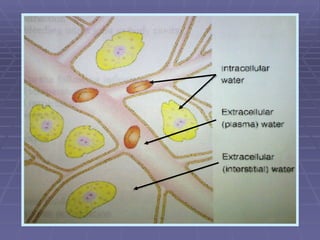
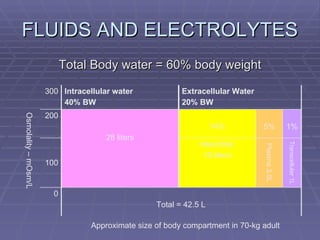







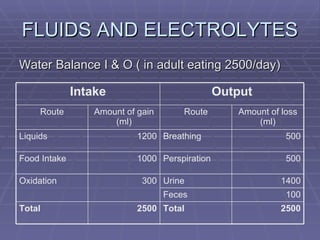






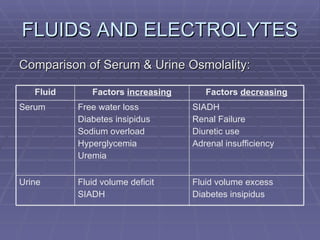






























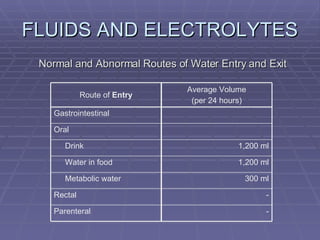


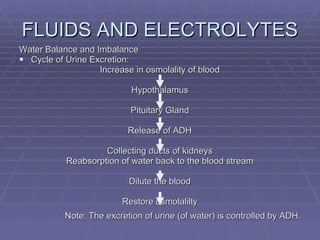



















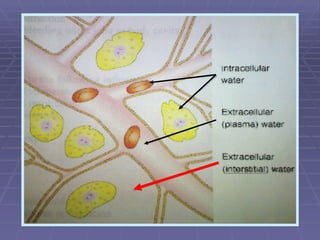
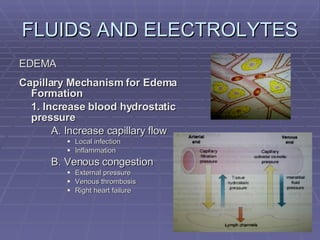
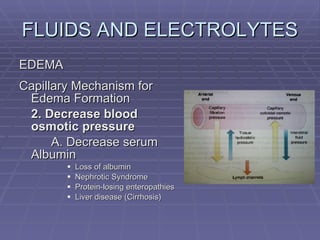
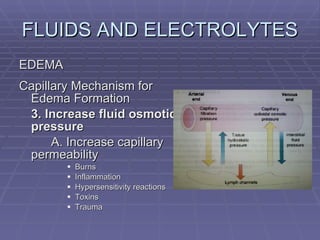
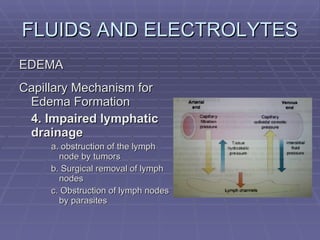













![FLUIDS AND ELECTROLYTES HYPERKALEMIA Serum K > 5.0 mEq/L Medical Therapy: To move K into cells (insulin, glucose, HCO 3 infusion) To counteract the cardiac effects of hyperkalemia (IV Ca gluconate) Remove K from the body (dialysis, diuretics, ion-exchange resins [Na polysterene sulfonate])](https://image.slidesharecdn.com/iv-therapy-1219127835671240-8/85/Intravenous-Therapy-IVF-Electrolytes-TPN-115-320.jpg)





























![Osmosis. Erythrocytes undergo no change in size in Isotonic solutions (A). There is increase in size in Hypotonic solutions (B) and decrease in size [shrink/crenate] in Hypertonic solution (C).](https://image.slidesharecdn.com/iv-therapy-1219127835671240-8/85/Intravenous-Therapy-IVF-Electrolytes-TPN-150-320.jpg)