HPLC- high performance liquid chromatography
- 1. Presented By: Prof. Hiren Thakkar Sigma Institute of Pharmacy Vadodara,Gujarat
- 2. INTRODUCTION Chromatography: a technique for the separation of a mixture by passing it in solution or suspension through a medium in which the components move at different rates. High performance liquid chromatography is a powerful tool in analysis, it yields high performance and high speed compared to traditional columns chromatography because of the forcibly pumped mobile phase. HPLC stands for “High-performance liquid chromatography”(sometimes referred to as High-pressure liquid chromatography). HPLC is a chromatographic technique that can separate a mixture of compounds. It is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of a mixture.
- 3. Stationary phase : The substance on which adsorption of the analyte (the substance to be separated during chromatography) takes place . It can be a solid, a gel, or a solid liquid combination Mobile phase : solvent which carries the analyte (a liquid or a gas) HPLC is a type of liquid chromatography where the sample is forced through a column that is packed with a stationary phase composed of irregularly or spherically shaped particles, a porous monolithic layer , or a porous membrane by a liquid (mobile phase) at high pressure.
- 4. PRINCIPLE High Performance Liquid Chromatography [HPLC] is principle is based on adsorption as well as partition chromatography is depending on the nature of stationary phase, if stationary phase is solid principle is based on adsorption chromatography and if stationary phase is liquid principle is based on partition chromatography. Adsorption chromatography - The affinity of molecules towards stationary phase is known as Adsorption chromatography. Partition chromatography - The molecule can moves in two phases of liquid is known as partition chromatography.
- 5. Continue.. When a mixture of components are introduced into the column . Various chemical and/or physical interactions take place between the sample molecules and the particles of the column packing . They travel according to their relative affinities towards the stationary phase. The component which has more affinity towards the adsorbent, travels slower. The component which has less affinity towards the stationary phase travels faster. Since no two components have the same affinity towards the stationary phase, the components are separated
- 7. When distribution of solute is equal between two phases , then effective distribution Kd =1 Individual zones adjacent to each other in the column where there is sufficient space for equal distribution of solutes in between stationary and mobile phase are called theoretical plates Length of theoretical plate in the column is called plate height More the theoretical plates , more efficient the separation 2000 or more theoretical plates are ideal for a column Components which have more affinity towards mobile phase elute first
- 8. TYPES OF HPLC SEPARATIONS Normal Phase: Separation of polar analytes by partitioning onto a polar, bonded stationary phase. Reversed Phase: Separation of non-polar analytes by partitioning onto a non-polar, bonded stationary phase. Adsorption: In Between Normal and Reversed. Separation of moderately polar analytes using adsorption onto a pure stationary phase (e.g. alumina or silica) Ion Chromatography: Separation of organic and inorganic ions by their partitioning onto ionic stationary phases bonded to a solid support. Size Exclusion Chromatography: Separation of large molecules based in the paths they take through a “maze” of tunnels in the stationary phase.
- 11. BASED ON PRINCIPLE OF SEPERATION Absorption Chromatography In the Absorption Chromatography solute molecules bond directly to the surface of the stationary phase the component which has more affinity towards mobile phase elutes first & the component which has less affinity towards stationary phase elutes later. No two components have same affinity towards mobile phase & stationary phase.
- 12. Ion-exchange chromatography Ion exchange chromatography is a process that allows the separation of ions and polar molecules based on their charge. It can be used for almost any kind of charged molecule including large proteins, small nucleotides and amino acids. Retention is based on the attraction between solute ions and charged sites bound to the stationary phase. Ions of the same charge are excluded. The use of a resin (the stationary solid phase) is used to covalently attach anions or cations onto it. Solute ions of the opposite charge in the mobile liquid phase are attracted to the resin by electrostatic forces.
- 13. Ion-pair chromatography It is a form of chromatography in which ions in solution can be "paired“ or neutralized and separated as an ion pair on a reversed- phase column. Ion-pairing agents are usually ionic compounds that contain a hydrocarbon chain that imparts a certain hydrophobicity so that the ion pair can be retained on a reversed-phase column.
- 14. Gel permeation chromatography This type of chromatography lacks an attractive interaction between the stationary phase and solute. The liquid or gaseous phase passes through a porous gel which separates the molecules according to its size. The pores are normally small and exclude the larger solute molecules, but allows smaller molecules to enter the gel, causing them to flow through a larger volume. This causes the larger molecules to pass through the column at a faster rate than the smaller ones.
- 15. Affinity Chromatography This is the most selective type of chromatography employed. It utilizes the specific interaction between one kind of solute molecule and a second molecule that is immobilized on a stationary phase. For example, the immobilized molecule may be an antibody to some specific protein. When solute containing a mixture of proteins are passed by this molecule, only the specific protein is reacted to this antibody, binding it to the stationary phase. This protein is later extracted by changing the ionic strength or pH.
- 17. BASED ON ELUTION TECHNIQUE 1.Isocratic elution A separation in which the mobile phase composition remains constant throughout the procedure is termed isocratic elution In isocratic elution, peak width increases with retention time linearly with the number of theoretical plates. This leads to the disadvantage that late-eluting peaks get very flat and broad. Best for simple separations Often used in quality control applications that support and are in close proximity to a manufacturing process
- 18. Gradient elution A separation in which the mobile phase composition is changed during the separation process is described as a gradient elution Gradient elution decreases the retention of the later-eluting components so that they elute faster, giving narrower peaks . This also improves the peak shape and the peak height Best for the analysis of complex samples Often used in method development for unknown mixtures Linear gradients are most popular
- 19. BASED ON SCALE OF OPERATION 1.Analytical HPLC No recovery of individual components of substance 2.Preparative HPLC Individual components of substance can be recovered
- 20. BASED ON TYPE OF ANALYSIS 1.Qualitative analysis Analysis of a substance in order to ascertain the nature of its chemical constituents We can separate individual components but cannot assess the quantity in this analysis 2.Quantitaive analysis Determining the amounts and proportions of its chemical constituents . Quantity of the impurity and individual components can be assessed
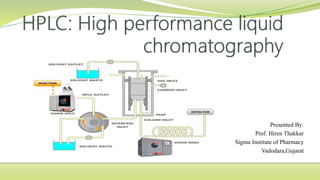
- 21. INSTRUMENTATION
- 22. Solvent delivery system(mobile phase) The mobile phase in HPLC refers to the solvent being continuously applied to the column or stationary phase The mobile phase acts as a carrier to the sample solution A sample solution is injected into the mobile phase of an assay through the injector port As a sample solution flows through a column with the mobile phase, the components of that solution migrate according to the non-covalent interaction of the compound with the column The chemical interaction of the mobile phase and sample , with the column , determine the degree of migration and separation of components contained in the sample The solvents or mobile phases used must be passed through the column at high pressure at about 1000 to 3000 psi. this is because as the particle size of stationary phase is around 5-10μ, so the resistance to the flow of solvent is high.
- 23. Pumps The role of the pump is to force a liquid (called the mobile phase) through the liquid chromatograph at a specific flow rate, expressed in milliliters per min (mL/min). Normal flow rates in HPLC are in the 1-to 2-mL/min range. Typical pumps can reach pressures in the range of 6000-9000 psi (400- to 600-bar). During the chromatographic experiment, a pump can deliver a constant mobile phase composition (isocratic) or an increasing mobile phase composition (gradient).
- 25. DISPLACEMENT PUMPS It consists of large, syringe like chambers equipped with a plunger activated by a screw driven mechanism powered by a stepping motor. So it is also called as Screw Driven Syringe Type Pump. Advantages:- It produces a flow that tends to be independent of viscosity & back pressure. Disadvantages:- It has a limited solvent capacity(~250) & considerably inconvenient when solvents must be changed.
- 26. RECIPROCATING PUMPS Consists of a small motor driven piston which moves rapidly back and forth in a hydraulic chamber that may vary from 35-400μL in volume On the back stroke , the separation column valve is closed , and the piston pulls in solvent from the mobile phase reservoir On the forward stroke, the pump pushes solvent out of the column from the reservoir A wide range of flow rates can be attained by altering the piston stroke volume during each cycle , or by altering the stroke frequency. Dual and triple head pump consists of identical piston chamber units which operate at 180 or 120 degrees out of phase(this system is significantly smoother because one pump is filling while the other is in the delivery cycle.
- 28. Syringe type pump These are most suitable for small bore columns because this pump delivers only a finite volume of mobile phase before it has to be refilled. These pumps have a volume between 250 to 500mL The pump operates by a motorized lead screw that delivers mobile phase to the column at a constant rate .The rate of solvent delivery is controlled by changing the voltage on the motor.
- 29. Constant pressure pump In these types of pumps , the mobile phase is driven through the column with the use of pressure from the gas cylinder A low-pressure gas source is needed to generate high liquid pressures The valving arrangement allows the rapid refill of the solvent chamber whose capacity is about 70mL This provides continuous phase flow rates
- 30. Injector: The injector serves to introduce the liquid sample into the flow stream of the mobile phase for analysis. It is equipped with six port valves so that a sample can be injected into the flow path at continuous pressure For a manual injector, the knob is manually operated to deliver the sample to the column The knob is set to LOAD position for sample injection using a syringe , the sample is injected into the sample loop , which is separated from the flow path The knob is turned to INJECT position and the eluent travels through the loop from the pump and delivers the sample to the column Typical sample volumes for manual injector are 5-to 20-microliters (μL). The injector must also be able to withstand the high pressures of the liquid system. An autos ampler is the automatic version for when the user has many samples to analyze or when manual injection is not practical. It can continuously Inject variable volume a of 1 μL – 1 mL
- 32. Column Considered the “heart of the chromatograph” the column’s stationary phase separates the sample components of interest using various physical and chemical parameters. It is usually made of stainless steel to withstand high pressure caused by the pump to move the mobile phase through the column packing other material include PEEK and glass The small particles inside the column are called the “packing” what cause the high back pressure at normal flow rates. Column packing is usually silica gel because of its particle shape , surface properties , and pore structure give us a good separation Other material used include alumina, a polystyrene-divinyl benzene synthetic or an ion- exchange resin – Pellicular particle: original, Spherical, nonporous beads, proteins and large biomolecules separation (dp: 5 μm) – Porous particle: common used, dp: 3 ~ 10 μm. Narrow size distribution, porous microparticle coated with thin organic film The dimensions of the analytical column are usually -straight, Length(5 ~ 25 cm), diameter of column(3 ~ 5 mm), diameter of particle(35 μm). Number (40 k ~ 70 k plates/m)
- 33. Guard column is used to remove particular matter and contamination, it protect the analytical column and contains similar packing its temperature is controlled at < 150 °C, 0.1 °C As mention before , columns are divided into different types according to their functions (see types of HPLC)
- 34. Detector: A chromatography detector is a device used in high performance liquid chromatography (HPLC) to detect components of the mixture being eluted off the chromatography column. The detector senses the presence of the individual components as they leave (elute) the column. The detectors converts a change in effluents into an electric signal that is recorded by data system.
- 35. IDEAL PROPERTIES OF A DETECTOR High sensitivity. Good stability and reproducibility. A linear response to solute. Negligible base line noise. Should be inexpensive. Capable of providing information on the identity of the solute. A short response time independent of flow-rate. The detector should be non-destructive. Responses independent of mobile phase composition.
- 36. The detectors used in HPLC are of majorly two types: Selective detectors (solute property) : These detectors respond to a particular physical or chemical property of the solute, being ideally independent of the mobile phase. They are as follow: Absorbance detectors Fluorescence detectors Electrochemical detectors Mass spectrometric detectors Universal detectors (bulk property): measure the difference in some physical property of the solute in the mobile phase compared to the mobile phase alone. They are generally universal in application but tend to have poor sensitivity and limited range. Such detectors are usually affected by even small changes in the mobile-phase composition which precludes the use of techniques such as gradient elution. They are as follow: Refractive index detectors Evaporating light scattering detectors
- 37. CLASSIFICATION OF HPLC DETECTORS UV/VIS - Fixed Wavelength - Variable Wavelength - Diode array (PDA) Refractive index -Deflection Detector -Refractive Detector (Fresnel refractometer) Fluorescence Detector Electrochemical Detector Conductivity Detector Evaporative light scattering (ELSD) Mass detector (LC-MS) IR detector Optical rotation detector (chiral detectors)
- 38. ULTRAVIOLET/VISIBLE SPECTROSCOPIC DETECTORS Measures the ability of solutes to absorb light at a particular wavelength(s) in the ultraviolet (UV) or visible (Vis) wavelength range. When light of a certain wavelength is directed at a flow cell, the substance inside the flow cell absorbs the light. As a result, the intensity of the light that leaves the flow cell is less than that of the light that enters it. An absorbance detector measures the extent to which the light intensity decreases (i.e., the absorbance).
- 39. Three Common types of UV/Vis Absorbance Detectors Fixed wavelength detectors Variable wavelength detectors Photodiode array detectors Fixed Wavelength Detector absorbance of only one given wavelength is monitored by the system at all times (usually 254 nm) Simplest and cheapest of the UV/VIS detectors Limited in flexibility Limited in types of compounds that can be monitored Variable Wavelength Detector a single wavelength is monitored at any given time, but any wavelength in a wide spectral range can be selected Wavelengths vary from 190-900 nm. More expensive, requires more advanced optics More versatile, used for a wider range of compounds More
- 40. Photo Diode Array Detector operates by simultaneously monitoring absorbance of solutes at several different wavelengths. Light from the broad emission source such as a deuterium lamp is collimated by an achromatic lens system so that the total light passes through the detector cell onto a holographic grating. In this way, the sample is subjected to light of all wavelengths generated by the lamp. The dispersed light from the grating is allowed to fall on to a diode array. The array may contain many hundreds of diodes and the output from each diode is regularly sampled by a computer and stored on a hard disc.
- 41. FLUORESCENCE DETECTOR It is based on the fluorescent radiation emitted by some compounds. The excitation source passes through the flow cell to a photo detector while a monochromatic measures the emission wavelengths. More sensitive and specific. The disadvantage is that most compounds are not fluorescent in nature. Fluorescence is a type of luminescence in which the light energy is released in the form of a photon in nanoseconds to microseconds.
- 42. REFRACTIVE INDEX DETECTOR Measures the overall ability of the mobile phase and its solutes to refract or bend light. Refractive index detector measures the molecule’s ability to deflect light in a flowing mobile phase in a flow cell relative to a static mobile phase contained in a reference cell. The amount of defection is proportional to the concentration of the solute in the mobile phase.
- 43. ELECTROCHEMICAL DETECTORS It is based on the measurement of the current resulting from an oxidation/ reduction reaction of the analyte at a suitable electrode. The level of current is directly proportional to the analyte concentration. Three electrodes are employed which are: Working electrode Auxiliary electrode Reference electrode
- 44. ELECTRICAL CONDUCTIVITY DETECTOR Used in analytical applications of ion-exchange chromatography for the detection of ionic compounds. Detector measures the ability of the mobile phase to conduct a current when placed in a flow-cell between two electrodes. Conductivity detectors measures electronic resistance and measured value is directly proportional to the concentration of ions present in the solution
- 45. EVAPORATIVE LIGHT SCATTERING DETECTOR (ELSD) Detection is based on the scattering of a beam of light by particles of compound remaining after evaporation of the mobile phase. It is a universal detector and does not required a compound to have a chromophore for detection There are three steps involved in detection: Nebulization Mobile phase evaporation Detection The flow from the column is nebulized with a stream of inert gas (Nebulize means to convert a liquid into a fine spray or mist). The mobile phase, whichmust be volatile, is evaporated, leaving tiny particles of the analytes (you can see why this detection method will not work with volatile compounds). The particles are passed through a laser beam and they scatter the laser light. The scattered light is measured at right angles to the laser beam by a photodiode detector.
- 47. MASS SPECTROMETER AS AN HPLC DETECTOR Liquid chromatography–mass spectrometry (LC-MS, or HPLC-MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography (or HPLC) with the mass analysis capabilities of mass spectrometry (MS). It is a method that combines separation power of HPLC with detection power of Mass spectrometry. Mass spectrometry (MS) is a powerful analytical tool that can supply both structural information about compounds and quantitative data relating to mass.
- 48. INFRARED SPECTROPHOTOMETRIC DETECTOR Infrared (IR) detectors have been used to a limited extent only for the analysis of non-polar lipids, with the specific absorbancefor the carbonyl function between 1650 and 1860 cm-1 (or at about 5.75 microns) being the spectral region of value. The wavelength scanning is provided by semicircular filter wedges, the wavelength range being from 4000- 690 cm-1 . It is not very sensitive and have serious drawback that most mobile phase solvents absorb strongly in the IR region
- 49. CHIRAL DETECTORS Chiral detectors are used for detection of optically active compounds such as amino acids, sugars, terpenes and other compounds containing an asymmetric carbon. There are two chiral detection techniques, polarimetry or optical rotary dispersion (ORD) and circular dichorism (CD). ORD detectors are based on differences in refractive index and CD detectors differentiate enantiomers by measuring differences between the absorption of light and left-handed circularly polarized light due to existence of a chiral chromophores.
- 50. RECORDERS AND INTEGRATORS Recorders are used to record responses obtained from the detectors after amplification, if necessary. They record the baseline & all the peaks obtained with respect tot time. Retention time can be found out from this recordings, but area under curve cannot be determined. The Integrators are improved versions of recorders with some data processing capabilities. hey can record the individual peaks with retention time, height, width of peaks, peak area, percentage area, etc. Integrators provides more information on peaks than recorders. In recent days computers and printers are used for recording and processing the obtained data & for controlling several operations.
- 51. Applications HPLC is one of the most widely applied analytical separation techniques. Pharmaceutical: Tablet dissolution of pharmaceutical dosages. Shelf life determinations of pharmaceutical products. Pharmaceutical quality control.
- 52. Environmental: •Phenols in Drinking Water. •Identification of diphenhydramine in sediment samples. •Biomonitoring of PAH pollution in high-altitude mountain lakes through the analysis of fish bile. •Estrogens in coastal waters - The sewage source. •Toxicity of tetracyclines and tetracycline degradation products to •environmentally relevant bacteria. •Assessment of TNT toxicity in sediment..
- 53. Forensics Identification of anabolic steroids in serum, urine, sweat and hair. Forensic analysis of textile dyes. Determination of cocaine and metabolites in meconium. Simultaneous quantification of psychotherapeutic drugs in human plasma.
- 54. Clinical Quantification of DEET in Human Urine. Analysis of antibiotics. Increased urinary excretion of aquaporin 2 in patients with liver cirrhosis. Detection of endogenous neuropeptides in brain extracellular fluids. Food and Flavor Ensuring soft drink consistency and quality. Analysis of vicinal diketones in beer. Sugar analysis in fruit juices. Polycyclic aromatic hydrocarbons in Brazilian vegetables and fruits. Trace analysis of military high explosives in agricultural crops. Stability of aspartame in the presence of glucose and vanillin
- 55. ADVANTAGES OF HPLC: 1.Separations fast and efficient (high resolution power) 2. Continuous monitoring of the column effluent 3. It can be applied to the separation and analysis of very complex mixtures 4. Accurate quantitative measurements. 5. Repetitive and reproducible analysis using the same column. 6. Adsorption, partition, ion exchange and exclusion column separations are excellently made. 7. HPLC is more versatile than GLC in some respects, because it has the advantage of not being restricted to volatile and thermally stable solute and the choice of mobile and stationary phases is much wider in HPLC 8. Both aqueous and non aqueous samples can be analyzed with little or no sample pre treatment 9. A variety of solvents and column packing are available, providing a high degree of selectivity for specific analyses. 10. It provides a means for determination of multiple components in a single analysis.
- 57. Thank You