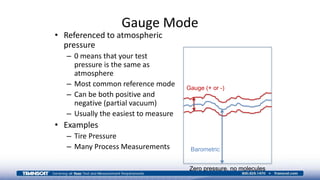
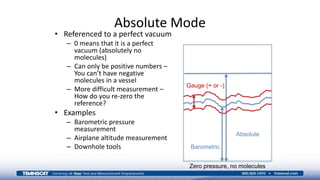
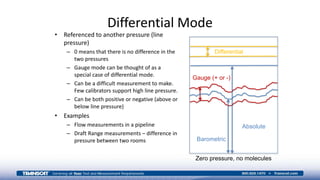
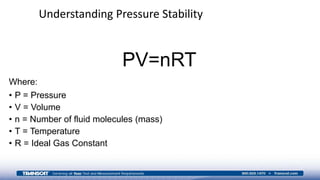
The document provides an overview of high-pressure calibration techniques and considerations, defining pressure and its reference modes: gauge, absolute, and differential. It emphasizes the importance of understanding pressure stability, influenced by factors like the number of fluid molecules, volume, and temperature, while also addressing safety, contamination, and equipment selection for high-pressure measurements. Examples of equipment include the Fluke 700HPPK pneumatic pressure pump and the P3100 hydraulic deadweight testers.