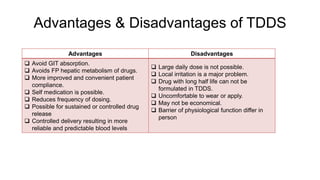
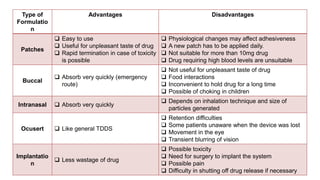
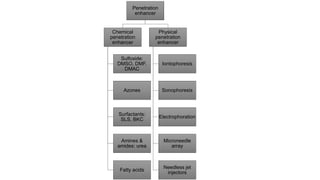
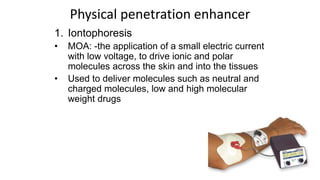
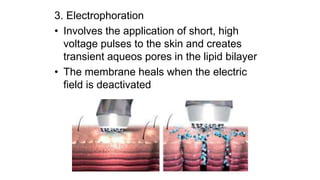
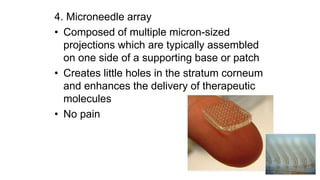
The document summarizes a new transdermal delivery system containing sumatriptan that is being developed. Due to production problems with sumatriptan tablets, the company is switching to sumatriptan capsules and developing the new transdermal system. As the new section head, the reader is responsible for ensuring the two new products meet standards of safety, quality and efficacy.