Poster_final_draft_July_23_2015
•
0 likes•48 views
This document summarizes research on producing activated carbon from lignin through chemical activation using potassium hydroxide (KOH). Lignin was obtained from Douglas fir forest residuals and mixed with KOH at different ratios before being carbonized at various temperatures. Nitrogen adsorption tests showed that pore volume, surface area, and pore size distribution depended on KOH ratio and temperature. Higher temperatures and KOH ratios resulted in more mesoporous carbon with larger surface areas, while lower temperatures produced microporous carbon. The optimal conditions were a KOH:lignin ratio of 3 and temperature of 750°C.
Report
Share
Report
Share
Download to read offline
Recommended
Presentation given by Professor Colin Snape from University of Nottingham on "Performance Enhanced Activated Spherical Carbon Adsorbents for CO2 Capture" in the Capture Technical Session on Solid Adsorption at the UKCCSRC Biannual Meeting - CCS in the Bigger Picture - held in Cambridge on 2-3 April 2014Performance Enhanced Activated Spherical Carbon Adsorbents for CO2 Capture - ...

Performance Enhanced Activated Spherical Carbon Adsorbents for CO2 Capture - ...UK Carbon Capture and Storage Research Centre
Elementar has developed the next generation of future-proof N/protein analyzers utilizing proprietary EAS REGAINER® technology and use alternative carrier gases, such as carbon dioxide for the rapid N exceed and argon for the rapid MAX N exceed, without sacrificing analysis performance.Helium-free, high performance N/protein analysis according to the Dumas princ...

Helium-free, high performance N/protein analysis according to the Dumas princ...Salm en Kipp bv Laboratoriumapparatuur
Recommended
Presentation given by Professor Colin Snape from University of Nottingham on "Performance Enhanced Activated Spherical Carbon Adsorbents for CO2 Capture" in the Capture Technical Session on Solid Adsorption at the UKCCSRC Biannual Meeting - CCS in the Bigger Picture - held in Cambridge on 2-3 April 2014Performance Enhanced Activated Spherical Carbon Adsorbents for CO2 Capture - ...

Performance Enhanced Activated Spherical Carbon Adsorbents for CO2 Capture - ...UK Carbon Capture and Storage Research Centre
Elementar has developed the next generation of future-proof N/protein analyzers utilizing proprietary EAS REGAINER® technology and use alternative carrier gases, such as carbon dioxide for the rapid N exceed and argon for the rapid MAX N exceed, without sacrificing analysis performance.Helium-free, high performance N/protein analysis according to the Dumas princ...

Helium-free, high performance N/protein analysis according to the Dumas princ...Salm en Kipp bv Laboratoriumapparatuur
Presentation given by Dr Hao Liu from University of Nottingham on "CO2 capture from NGCC Flue Gas and Ambient Air Using PEI-Silica Adsorbent" in the Capture Technical Session on Solid Adsorption at the UKCCSRC Biannual Meeting - CCS in the Bigger Picture - held in Cambridge on 2-3 April 2014CO2 capture from NGCC Flue Gas and Ambient Air Using PEI-Silica Adsorbent - D...

CO2 capture from NGCC Flue Gas and Ambient Air Using PEI-Silica Adsorbent - D...UK Carbon Capture and Storage Research Centre
Densities and Refractive Indices of solutions of
potassium bromate (KBrO3) have been studied in water and
0.1%, 0.2%, 0.3%, 0.4% and 0.5% (w/v) aqueous solution of
KCl with temperature in the range T = 298.15˚K- 313.15˚K. The
data obtained is utilized to determine Specific Refraction (RD)
and Molar Refraction (RM) of solutions. The values of Refractive
indices, Molar Refraction (RM) and Molar Polarisability (α)
constant are found to be decreased with decreasing concentration
of solute in solvent and these results are also interpreted in terms
of interaction in salt solution. It has been verified that Molar
Refraction is additive and constitutive property. STUDY OF MOLAR REFRACTION AND POLARISABILITY CONSTANT OF AQUEOUS SOLUTIONS OF...

STUDY OF MOLAR REFRACTION AND POLARISABILITY CONSTANT OF AQUEOUS SOLUTIONS OF...International Journal of Technical Research & Application
More Related Content
What's hot
Presentation given by Dr Hao Liu from University of Nottingham on "CO2 capture from NGCC Flue Gas and Ambient Air Using PEI-Silica Adsorbent" in the Capture Technical Session on Solid Adsorption at the UKCCSRC Biannual Meeting - CCS in the Bigger Picture - held in Cambridge on 2-3 April 2014CO2 capture from NGCC Flue Gas and Ambient Air Using PEI-Silica Adsorbent - D...

CO2 capture from NGCC Flue Gas and Ambient Air Using PEI-Silica Adsorbent - D...UK Carbon Capture and Storage Research Centre
Densities and Refractive Indices of solutions of
potassium bromate (KBrO3) have been studied in water and
0.1%, 0.2%, 0.3%, 0.4% and 0.5% (w/v) aqueous solution of
KCl with temperature in the range T = 298.15˚K- 313.15˚K. The
data obtained is utilized to determine Specific Refraction (RD)
and Molar Refraction (RM) of solutions. The values of Refractive
indices, Molar Refraction (RM) and Molar Polarisability (α)
constant are found to be decreased with decreasing concentration
of solute in solvent and these results are also interpreted in terms
of interaction in salt solution. It has been verified that Molar
Refraction is additive and constitutive property. STUDY OF MOLAR REFRACTION AND POLARISABILITY CONSTANT OF AQUEOUS SOLUTIONS OF...

STUDY OF MOLAR REFRACTION AND POLARISABILITY CONSTANT OF AQUEOUS SOLUTIONS OF...International Journal of Technical Research & Application
What's hot (20)
Remedition Using Soy Based Aerobic Co-Metabolism for Removal of Chlorinated H...

Remedition Using Soy Based Aerobic Co-Metabolism for Removal of Chlorinated H...
Gasification Performance Improvement of Treated SRF Residue by Using Minerals...

Gasification Performance Improvement of Treated SRF Residue by Using Minerals...
Room-temperature synthesis of 3-dimentional Ag-graphene hybrid hydrogel with ...

Room-temperature synthesis of 3-dimentional Ag-graphene hybrid hydrogel with ...
CO2 capture from NGCC Flue Gas and Ambient Air Using PEI-Silica Adsorbent - D...

CO2 capture from NGCC Flue Gas and Ambient Air Using PEI-Silica Adsorbent - D...
A study on fixed bed gasification of treated solid refuse fuel residue

A study on fixed bed gasification of treated solid refuse fuel residue
Undergraduate Inorganic Laboratory Development: Epoxides & CO2 Coupling Under...

Undergraduate Inorganic Laboratory Development: Epoxides & CO2 Coupling Under...
STUDY OF MOLAR REFRACTION AND POLARISABILITY CONSTANT OF AQUEOUS SOLUTIONS OF...

STUDY OF MOLAR REFRACTION AND POLARISABILITY CONSTANT OF AQUEOUS SOLUTIONS OF...
CO2 to fuels and chemicals course material final version

CO2 to fuels and chemicals course material final version
Similar to Poster_final_draft_July_23_2015
Activated carbon monoliths (ACMs) with average pore diameters in the meso- and micropore regions were successfully produced from biomass material. ACM synthesis uses chemical activation with KOH and ZnCl2 activating agents. The carbon and activating agent mass ratios were 1:1, 1:3, 1:5 and 1:7. Both activating materials produced an ACM with an average pore diameter of 3.2 nm. The specific capacitance, specific surface area, energy and power were as high as 63 F/g, 650 m2/g, and 0.23 Wh/kg for KOH and 73 F/g, and 522 m2/g, and 19 W/kg for ZnCl2 activating agents, respectively. For comparison, we also studied the physical and electrochemical properties of ACM with an average pore size in the micropore range from the same raw material.Meso- and Microporous Carbon Electrode and Its Effect on the Capacitive, Ene...

Meso- and Microporous Carbon Electrode and Its Effect on the Capacitive, Ene...International Journal of Power Electronics and Drive Systems
Batch adsorption experiments were carried out for
the adsorption of cationic dye from aqueous solution onto
composite activated carbon. The composite activated carbon was
prepared from brewer’s spent grain and sea bean shell at a ratio
of 1:1. The equilibrium studies were done at different
concentrations and temperatures. The equilibrium data were
fitted to Langmuir, Freundlich, Dubinin-Radushkevich, and
Temkin isotherm models. The results showed that both Lagmuir
and Freundlich isotherm model fitted the data reasonably well
but Freundlich isotherm fitted better in the temperature range
studied. This confirmed that the adsorption is heterogeneous,
non-specific and non-uniform in nature. Kinetic studies were also
undertaken in terms of first order, second order, pseudo first
order, pseudo second order, Elovich, Boyd, and intra-particle
diffusion models. The results indicated that the data followed
pseudo second order model with surface adsorption and intraparticle
diffusion concurrently operating during the adsorbateadsorbent
interaction. The values of the thermodynamic
parameters computed from Van’t Hoff plot confirmed the
process to be endothermic and spontaneous in nature. EQUILIBRIUM, KINETIC AND THERMODYNAMIC STUDIES ON BASIC DYE ADSORPTION USING ...

EQUILIBRIUM, KINETIC AND THERMODYNAMIC STUDIES ON BASIC DYE ADSORPTION USING ...International Journal of Technical Research & Application
Similar to Poster_final_draft_July_23_2015 (20)
Utilization of biodiesel wastes as a bioresource for the preparation of activ...

Utilization of biodiesel wastes as a bioresource for the preparation of activ...
Thermal regeneration of activated carbon saturated with nitrate ions from an ...

Thermal regeneration of activated carbon saturated with nitrate ions from an ...
The characteristic of pelleted broiler litter biochar derived from pilot scal...

The characteristic of pelleted broiler litter biochar derived from pilot scal...
Preparation and Characterization of Activated Carbon from Hura Crepitans Linn...

Preparation and Characterization of Activated Carbon from Hura Crepitans Linn...
Meso- and Microporous Carbon Electrode and Its Effect on the Capacitive, Ene...

Meso- and Microporous Carbon Electrode and Its Effect on the Capacitive, Ene...
Undergraduate Laboratory Development: Finding Cost-Effective Catalysts for th...

Undergraduate Laboratory Development: Finding Cost-Effective Catalysts for th...
Application of micromechanics on alkali activated materials

Application of micromechanics on alkali activated materials
Enhanced fluidized bed methanation over a Ni Al2O3 catalyst for production of...

Enhanced fluidized bed methanation over a Ni Al2O3 catalyst for production of...
2014_Nguyen et al._The Journal of Supercritical Fluids

2014_Nguyen et al._The Journal of Supercritical Fluids
Energy Recovery of Biomass: Study Comparative Experimental of Fixed Bed Combu...

Energy Recovery of Biomass: Study Comparative Experimental of Fixed Bed Combu...
Energy Recovery of Biomass: Study Comparative Experimental of Fixed Bed Combu...

Energy Recovery of Biomass: Study Comparative Experimental of Fixed Bed Combu...
Removal of Coke during Steam Reforming of Ethanol over La-CoOx Catalyst

Removal of Coke during Steam Reforming of Ethanol over La-CoOx Catalyst
Performance of Graphene-based Supercapacitors with Different Mass Ratio of FC...

Performance of Graphene-based Supercapacitors with Different Mass Ratio of FC...
Modification of CWZ-22 with KOH to enhance CO2 adsorption

Modification of CWZ-22 with KOH to enhance CO2 adsorption
2014_Belkheiri et al._Cellulose Chemistry and Technology

2014_Belkheiri et al._Cellulose Chemistry and Technology
Material Science and Engineering-B_Synthesis of ultra high molecular weight p...

Material Science and Engineering-B_Synthesis of ultra high molecular weight p...
EQUILIBRIUM, KINETIC AND THERMODYNAMIC STUDIES ON BASIC DYE ADSORPTION USING ...

EQUILIBRIUM, KINETIC AND THERMODYNAMIC STUDIES ON BASIC DYE ADSORPTION USING ...
Poster_final_draft_July_23_2015
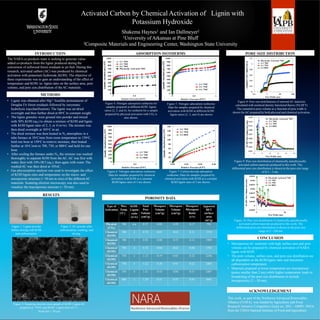
- 1. Activated Carbon by ChemicalActivation of Lignin with Potassium Hydroxide Shakema Haynes1 and Ian Dallmeyer2 1University ofArkansas at Pine Bluff 2Composite Materials and Engineering Center, Washington State University INTRODUCTION The NARAco-products team is seeking to generate value- added co-products from the lignin produced during the conversion of softwood forest residuals to jet fuel. During this research, activated carbon (AC) was produced by chemical activation with potassium hydroxide (KOH). The objective of these experiments was to gain an understanding of the effect of temperature and KOH- to- lignin ratio on the surface area, pore volume, and pore size distribution of theAC materials. METHODS RESULTS This work, as part of the Northwest Advanced Renewables Alliance (NARA), was funded byAgriculture and Food Research Initiative Competitive Grant no. 2011 – 68005- 30416 from the USDANational Institute of Food andAgriculture. Figure 4: Nitrogen adsorption isotherms for samples prepared at different KOH :lignin ratios (2, 3, and 4).An isotherm for a sample prepared by physical activation with CO2 is also shown. Figure 5: Nitrogen adsorption isotherms: Data for samples prepared by chemical activation with KOH at different KOH: lignin ratios (2, 3, and 4) are shown Figure 6: Nitrogen adsorption isotherms: Data for samples prepared by chemical activation with KOH at a constant KOH:lignin ratio of 3 are shown. Figure 7: Carbon dioxide adsorption isotherms: Data for samples prepared by chemical activation with KOH at a constant KOH:lignin ratio of 3 are shown. Figure 8: Pore size distributions of selected AC materials calculated with nonlocal density functional theory (NLDFT) The cumulative pore volume as a function of pore width is shown forAC prepared by both physical and chemical activation. Figure 9: Pore size distribution of chemically and physically activated carbon materials produced in this work. The differential pore size distribution is shown in the pore size range of 0.3 – 5 nm. ADSORPTION ISOTHERMS PORE SIZE DISTRIBUTION Figure 10: Pore size distribution of chemically and physically activated carbon materials produced in this work. The differential pore size distribution is shown in the pore size range of 5 - 100 nm. • Lignin was obtained after Mg2+ bisulfite pretreatment of Douglas Fir forest residuals followed by enzymatic hydrolysis (saccharification). The lignin was air-dried overnight and then further dried at 60oC to constant weight. • The lignin granules were ground into powder and mixed with 50% KOH (aq.) to obtain a mixture of KOH and lignin with KOH:lignin ratio of 2, 3, or 4 (w/w). The mixture was then dried overnight at 105oC in air. • The dried mixture was then heated in N2 atmosphere in a tube furnace at 10oC/min from room temperature to 150oC, held one hour at 150oC to remove moisture, then heated further at 10oC/min to 700, 750, or 800oC and held for one hour. • After cooling the furnace under N2, the mixture was washed thoroughly to separate KOH from theAC.AC was first with water, then with 10% HCl (aq.), then again with water. The washedAC was then dried at 105oC. • Gas physisorption analysis was used to investigate the effect of KOH:lignin ratio and temperature on the micro- and mesoporous structure (< 50 nm in size) of the differentAC materials. Scanning electron microscopy was also used to visualize the macroporous structure (> 50 nm). Figure 1: Lignin powder before mixing with KOH and carbonization. Figure 2:AC powder after carbonization, washing, and drying. Figure 3: Scanning electron micrograph of KOH: LigninAC prepared at 750oC and KOH: Lignin ratio of 3:1. Scale bar = 30 µm POROSITY DATA CONCLUSION • MicroporousAC materials with high surface area and pore volume can be prepared by chemical activation of NARA lignin with KOH. • The pore volume, surface area, and pore size distribution are all dependent on the KOH:lignin ratio and maximum carbonization temperature. • Materials prepared at lower temperature are microporous (pores smaller than 2 nm) while higher temperature leads to broadening of the pore size distribution to include mesoporosity (2 – 50 nm). ACKNOWLEDGEMENT Type of Activation Max. Temp. (oC) KOH: Lignin ratio (w/w) Total Pore Volume (cm3/g) Mesopore Volume (cm3/g) Micropore Volume (cm3/g) Mesopore/ Micropore Ratio (v/v) Apparent BET surface area (m2/g) Physical (CO2) 700 n/a 0.37 0.05 0.29 0.17 791 Chemical (KOH) 700 2 0.78 0.07 0.63 0.11 1719 Chemical (KOH) 700 3 0.92 0.08 0.73 0.11 1989 Chemical (KOH) 700 4 0.74 0.04 0.62 0.06 1705 Chemical (KOH) 750 2 1.13 0.19 0.83 0.23 2236 Chemical (KOH) 750 3 1.22 0.20 0.91 0.22 2405 Chemical (KOH) 750 4 1.41 0.43 0.84 0.51 2487 Chemical (KOH) 800 3 1.20 0.51 0.55 0.93 1691