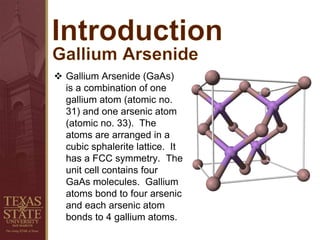
This document discusses gallium arsenide (GaAs), including its history, properties, manufacturing processes, applications, and cost. GaAs is a compound of gallium and arsenic atoms arranged in a cubic lattice. It has excellent electronic properties such as high electron mobility and saturated velocity. Its direct bandgap allows efficient light emission, making it useful for solar cells and optoelectronics. GaAs is manufactured through processes like Czochralski growth and epitaxy to produce wafers for devices. Though expensive, GaAs has applications in areas like wireless communication, satellites, and solar energy due to its high performance capabilities.