Atomic_Structure.pptx
•Download as PPTX, PDF•
0 likes•1 view
Chemistry notes
Report
Share
Report
Share
Recommended
More Related Content
Similar to Atomic_Structure.pptx
Similar to Atomic_Structure.pptx (20)
B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...

B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...
EE2317-Course- 02 Atomic Structures and Interatomic bonding.pdf

EE2317-Course- 02 Atomic Structures and Interatomic bonding.pdf
8th Grade - Chapter 16 - Atomic Structure and Chemical Bonding

8th Grade - Chapter 16 - Atomic Structure and Chemical Bonding
Recently uploaded
APM Welcome
Tuesday 30 April 2024
APM North West Network Conference, Synergies Across Sectors
Presented by:
Professor Adam Boddison OBE, Chief Executive Officer, APM
Conference overview:
https://www.apm.org.uk/community/apm-north-west-branch-conference/
Content description:
APM welcome from CEO
The main conference objective was to promote the Project Management profession with interaction between project practitioners, APM Corporate members, current project management students, academia and all who have an interest in projects.APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across SectorsAssociation for Project Management
Recently uploaded (20)
Z Score,T Score, Percential Rank and Box Plot Graph

Z Score,T Score, Percential Rank and Box Plot Graph
9548086042 for call girls in Indira Nagar with room service

9548086042 for call girls in Indira Nagar with room service
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
Web & Social Media Analytics Previous Year Question Paper.pdf

Web & Social Media Analytics Previous Year Question Paper.pdf
APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across Sectors
Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...

Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...
BAG TECHNIQUE Bag technique-a tool making use of public health bag through wh...

BAG TECHNIQUE Bag technique-a tool making use of public health bag through wh...
Measures of Central Tendency: Mean, Median and Mode

Measures of Central Tendency: Mean, Median and Mode
Atomic_Structure.pptx
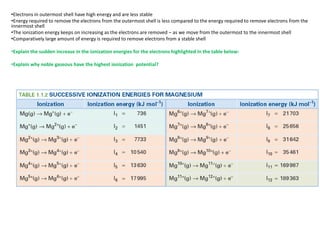
- 1. •Electrons in outermost shell have high energy and are less stable •Energy required to remove the electrons from the outermost shell is less compared to the energy required to remove electrons from the innermost shell •The ionization energy keeps on increasing as the electrons are removed – as we move from the outermost to the innermost shell •Comparatively large amount of energy is required to remove electrons from a stable shell •Explain the sudden increase in the ionization energies for the electrons highlighted in the table below: •Explain why noble gaseous have the highest ionization potential?
- 2. 1. Explain why the first ionization energies of Lithium, Sodium and Potassium is low? 2. Explain why the noble gases have the highest ionization energies?
- 3. Na (11) (2,8,1) (1s2, 2s2, 2P6, , 3s1 )
- 4. S orbital can accommodate maximum 2 electrons P orbital can accommodate maximum 6 electrons Energy s<p<d<F • Electrons have wave like properties • S orbital, p orbital • S orbital can accommodate maximum 2 electrons • P orbital can accommodate maximum 6 electrons • Pauli’s exclusion principle • Heisenberg uncertainty principle
- 5. 1s 2s 2p 3s 3p 4s S orbital 2 electrons P orbital 6 electrons Write the electronic configuration for Be (4) Mg(12) Al (13) Li (3) Na(11) C(6) 0(8) F(9) N(5) Ne(10) Ar(18)