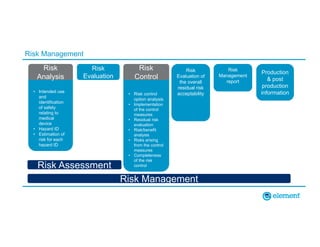
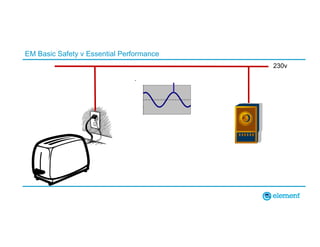
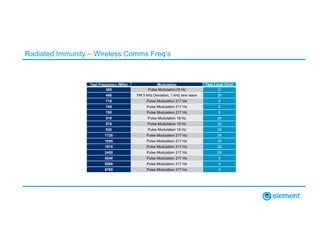
The document outlines the updates in IEC 60601-1-2:2014, particularly regarding electromagnetic compatibility and risk management in medical electrical equipment. It discusses the structure of standards, risk evaluation, and the safety performance requirements necessary for various medical devices. Additionally, it highlights changes from previous editions, including testing protocols for electromagnetic disturbances in defined environments.