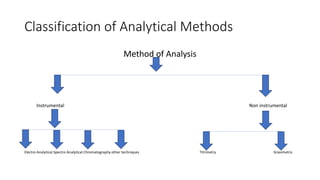
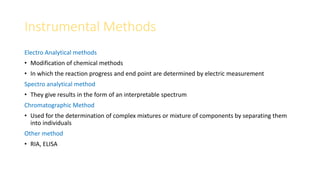
Pharmaceutical analysis involves qualitative, quantitative, and semi-quantitative analysis to determine what substances are present in a sample and how much of each substance is present. There are various types of analytical methods including classical methods like titrimetry and gravimetry as well as instrumental methods using techniques like spectroscopy, chromatography, and electroanalytical methods. The document discusses the scope, classification, advantages, and limitations of different pharmaceutical analytical methods.