unit-II (Acid base titration).pptx
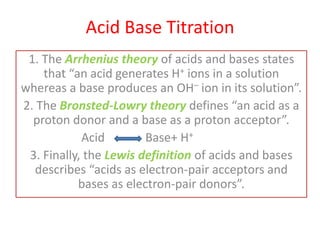
- 1. Acid Base Titration 1. The Arrhenius theory of acids and bases states that “an acid generates H+ ions in a solution whereas a base produces an OH– ion in its solution”. 2. The Bronsted-Lowry theory defines “an acid as a proton donor and a base as a proton acceptor”. Acid Base+ H+ 3. Finally, the Lewis definition of acids and bases describes “acids as electron-pair acceptors and bases as electron-pair donors”.
- 2. An acid-base titration is a quantitative analysis of acids and bases; through this process, an acid or base of known concentration neutralizes an acid or base of unknown concentration. The titration progress can be monitored by visual indicators, pH electrodes, or both. The reaction’s equivalence point is the point at which the titrant has exactly neutralized the acid or base in the unknown analyte; if you know the volume and concentration of the titrant at the equivalence point, you can calculate the concentration of a base or acid in the unknown solution.
- 3. Example: The pH range of phenolphthalein is 8.0 – 9.8. In solutions with pH less than 8.0, it is colorless and in solutions with pH of more than 9.8, its color is pink. Phenolphthalein is colorless in weak alkaline solution and pink in strong alkaline solution “Acid base indicators are those substances which have one color in acidic solution and another color in alkaline solution i.e. their color changes with proper change in pH value.”
- 4. It is clear that it is not necessary that the color of an acid base indicator is the same in all alkaline solutions. Likewise, it is also not necessary that the color of an acid base indicator be the same in all acidic solution Theory of Indicators: (explain the behavior of acid base indicators.) 1. Ostwarld Theory 2. Quinonoid Theory
- 5. Ostwarld Theory Theory of indicator action was suggested by W.ostwald. Most of acid base indicator are weak organic acids or bases & colour changes due to ionization of indicator The unionized indicator has different colour than ionized indicator Example: phenolphthalein is a weak acid. It is colorless. On dissolving it in water, it is ionized into colorless H ions and pink anions Consider HPh is weak organic acid indicator & in aqueous solution it is well dissociate HPh(colorless) ⇋ H(colorless) + Ph(Pink) (undissociated) (dissociated) In the above reaction, dissociated indicator have different colour than undissociated indicator
- 6. In acid base titration , if the indicator placed in acidic solution of acid HA, following equilibrium exist HPh ⇋ H+ + Ph- HA ⇋ H+ + A- Hph is a weak acid ,so it poorly dissociate & due to common ion effect the dissociation further decreased. The equilibrium is shifts towards left side. Therefore in presence of acid the dissociation of Hph is almost negligible & indicator is mostly in undissociated form & show undissociated indicator colour ( colourless)
- 7. But if the same indicator added in alkali solution of base BOH, the dissociation of Hph will be increased HPh ⇋ H+ + Ph- (dissociation increased) BOH ⇋ OH- + B+ The hydrogen ion removed by OH- by formation of water molecules & equilibrium is shifted towards right side & concentration of Ph- will more than Hph which give dissociated indicator colour. i.e phenolphthalein give pink colour
- 8. Similarly if the indicator is weak organic base , it poorly dissociated in alkali solution but dissociation increased in acidic solution. Example : Thus the color change of methyl orange can also be explained. Methyl orange is a weak base and thus dissociates – MeOH(Yello) ⇋ Me+(Red) + OH-(colorless) In basic medium: Due to the common ion effect in the presence of OH ions in alkaline medium, ionization of MeOH is very low, i.e the concentration of Me is very low and the color of the solution remains yellow.
- 9. MeOH ⇋ Me ++ OH- HCl ⇋ H ++ Cl- H ++ OH- ⇋ H2O Me+ + Cl- ⇋ MeCl
- 10. Quinonoid theory Quinonoid theory suggest that the colour change of indicator is due to structural changes of Indicator. In acidic solution indicator exist in different structural form & in basic solution it exist in different structural form These structural form have different colours, therefore in acidic solution indicator give different colour and in basic solution different colour.
- 11. According to this theory, indicator are aromatic carbonic compounds used in acid base titrations. They are a mixture of at least two movable forms Among these, one form is in acidic medium and the other form in alkaline medium in greater proportion. These two forms are called benzenoid and quinonoid forms. Both these forms have different colors. The color of the quinonoid form is darker than the color of the benzenoid form
- 12. Therefore, when the pH value of a solution changes, there is a change in the benzenoid form to the quinonoid form or the quinonoid form to the benzenoid form causing a change in color.
- 13. Example: The acidic solution of phenolphthalein, being in its benzenoid form, is colorless. When this solution is added to such a base that the solution becomes alkaline, the entire benzenoid form turns into a quinonoid form. The color of phenolphthalein’s alkaline solution also becomes red as the color of the anions obtained from the quinonoid form is red.
- 14. Similarly methyl orange is also found in two tautomeric forms. In acidic solution, this compound remains in quinonoid form and in alkaline solution it remains in benzenoid form. The color of quinonoid form is red and the color of benzenoid form is yellow orange. Therefore, the color of acidic solution of methyl orange is red and the color of alkaline solution is yellow orange.
- 15. Classification of acid base Titration 1. Titration of strong acid with strong base 2. Titration of weak acid with strong base 3. Titration of weak base with strong acid 4. Titration of weak base with weak acid 5. Titration of polybasic acid with strong base
- 26. H a n
- 27. Neutralization curve Neutralization curve represents the neutralization reaction between acid & base in neutralization titration. It is plotted between the pH and volume of acid or base added. The following information derived from neutralization curve 1. The curve is useful in studying the neutralization process by studying the change in hydrogen ion concentration during titration 2. It represent the progress of acid base titration 3. It indicate the end point of titration 4. It indicate the sensitivity of titration & chances of error 5. On the basis of pH conditions near inclination point the selection of indicator for particular titration is made.
- 28. Non Aqueous Titration Non aqueous titration are the titration which carried out using non aqueous solvent (other than water) Why choose non aqueous titration: 1. If the reactants or productants are insoluble in water. 2. React with water 3. Too weak base or acid that cannot be titrated in water due to their poor infliction in pH at end point in water Example of non-aqueous solvent: - Acetone - Glacial acetic acid - Formic acid - Ethylene diamine - chloroform
- 29. Non aqueous solvent 1. Protophilic solvents: These are the solvent which have high affinity for proton & are basic in nature. HB + S SH+ + B- Acid solvent solvated proton conjugate base Example: Liquid ammonia, ketones and amines 2. Protogenic solvents: which readily donate protons & are acidic in nature Example: sulphuric acid, hydrogen fluoride 3. Amphiprotic solvents: Solvents which are able to donate as well as accept proton( Protogenic & protophilic solvent) Example: Acetic acid CH3COOH CH3COO- + H+ While in presence of strong acid like perchloric acid, it act as base & accept proton CH3COOH + HClO4 CH3COOH2 + ClO4 4. Aprotic solvent: solvents which are chemically inert & does not donate or accept proton , therefore they don't have any basic or acidic nature. Example: Toluene, chloroform, benzene etc.
- 30. ALKALIMETRY & ACIDIMETRY Alkalimetry refers to the determination of strength of basic substances by titrating them with standard acid solution. Acidimetry refers to the determination of strength of acidic substances by titrating them with standard base solution. In non-aqueous titration weak base & weak acid are determined.
- 31. Titration of weak base: Titration of weak base done by standard acid mainly perchloric acid. Acetic acid used as solvent for dissolving weak base. Reaction involved can be explained as follows.