Spectroscopy chapter 9 textbook of organic chemistry arun bahl-
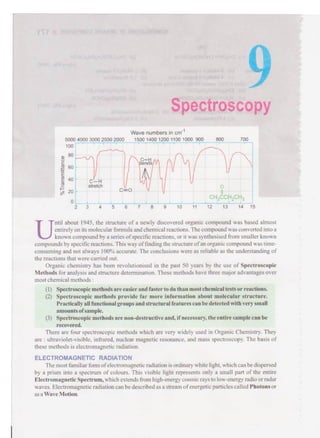
- 1. Spectroscopy C-H stretch Wave numbers in cm"1 5000 4000 3000 2500 2000 1500 1400 1200 1100 1000 900 800 4 r/ 700 0 ch3cch2ch3 2 3 4 5 6 7 8 9 10 11 12 13 14 15 ntil about 1945, the structure of a newly discovered organic compound was based almost entirely on itsmolecular formula andchemicalreactions.The compoundwas convertedinto a knowncompound by a series ofspecific reactions,or it was synthesised from smaller known compoundsby specific reactions.This way offinding the structure ofan organic compoundwas time- consuming and not always 100% accurate. The conclusions were as reliable as the understanding of the reactions that were carried out. Organic chemistry has been revolutionised in the past 50 years by the use of Spectroscopic Methods for analysis and structure determination. These methods have three major advantages over most chemical methods : (1) Spectroscopic methods are easier and faster to do than mostchemicaltests or reactions. (2) Spectroscopic methods provide far more information about molecular structure. Practicallyallfunctional groups andstructural features can bedetectedwithvery small amounts ofsample. (3) Spectroscopic methods are non-destructive and,ifnecessary,the entiresample can be There are four spectroscopic methods which are very widely used in Organic Chemistry. They are :ultraviolet-visible, infrared, nuclear magnetic resonance, and mass spectroscopy. The basis of these methods is electromagnetic radiation. ELECTROMAGNETIC RADIATION The most familiar form ofelectromagneticradiation is ordinary white light,which canbedispersed by a prism into a spectrum of colours. This visible light represents only a small part of the entire Electromagnetic Spectrum,which extends fromhigh-energycosmic raysto low-energy radioor radar waves. Electromagnetic radiationcan bedescribed as a stream ofenergetic particles called Photonsor as aWave Motion. recovered.
- 2. SPECTROSCOPY ÿ 173 ESE8Z2 10"Hz 10'5Hz 1010Hz Cosmic X-rays (UV) (UV) (Vis) (IR) (IR) Microwave and Vacuum Near Visible Near Infrared and 7-rays ultraviolet ultraviolet infrared radio 0.1 nm 11m ouvim 2 »im 50 nm Fig. 9.1. Electromagnetic spectrum. The fundamental equations of electromagnetic radiationare : E= hv and vA =c where E is the energy of radiation, h is Planck's constant, v (pronounced as nu) is the frequency of radiation, A (pronounced as lambda) is the wavelength ofthe radiation, andc isthe velocity of light which isconstant. Since v and A arc relatedbya constant (c),the energy of radiationcan be described by either frequency or the wavelength. Fig. 9.1 shows the various components of the electromagnetic spectrum. Inorganic chemistry, the importantwavelength regions are the ultraviolet,visible, and infrared. The ultraviolet and visible regions are adjacent, and the boundry is somewhat arbitrary since it depends upon humanperceptionoflight.The commonlyusedunitfor wavelength inthisregion isthe Nanometer ( 1 nm= 10~9 meter =0.1A, angstrom).The infraredregionisat longer wavelengths (lower energy,sinceE=heIX).Forthis region,the scalcmaybe expressedinwavelength (microns,[!)or Wave Number. The latter is generally preferred. The wave number is the reciprocal of the wavelength in centimetres. PRINCIPLEOF SPECTROSCOPY All organic compounds interact with electromagnetic radiation, that is, they absorb energy. When a moleculeabsorbs energy,a transformation occursthat may becither temporary or permanent. Lower-energy radiation may cause a molecularrotationor a bondvibration. Higher-energy radiation may cause the promotion of electrons to higher energy levels or bond clcavagc. Whether the transformation involvesmolecular rotation,bondvibration, or electronic transition. the molecule absorbs only the wavelength of radiation with exactly the energy necessary for the transition. The absorption of wavelengths of radiation is selective for a particular transition which depends on the structure ofthe molecule.By measuringthe absorptionspectra of knowncompounds. we can correlate the wavelengths of energy absorbed with characteristic structural features. This information is then used to determine the structure of unknown compounds. SPECTROPHOTOMETER The instrument that is used to measure the amount ofelectromagnetic radiation absorbed by an organic moleculeiscalled Spectrophotometer or Spectrometer. Itconsistsofa lightsource ofradiation. with a prism that can select the desired wavelengths, which are passed through a sample of the compoundbeinginvestigated(Fig.9.2). The radiationthat isabsorbed bythe sample can be detected, analysed, and recorded. The recording is called the Spectrum of the compound. I I
- 3. 174ÿ A TEXTBOOK OF ORGANIC CHEMISTRY ivd_ Light Prism source selector Sample Detector Recorder Fig. 9.2. Basic components of a spectrophotometer. INFRARED SPECTROSCOPY An infrared(IR) spectrometer subjects a compound to infrared radiation in the 5000-667 cm'1 (2|im) range. Although this radiation is weak, it does supply sufficient energy for bonds in the moleculeto vibrate by Stretchingor Bending(Fig.9.3).The atoms ofamoleculecanbeconsideredas linkedby springs that are set inmotion by the applicationof energy. As the molecule is subjected to the individual wavelengths in the 5000-667 cm"1 range, it absorbs only those possessing exactly the energy requiredto cause a particular vibration. Energy absorptions are recorded as bands (peaks) on V V V V »Stretching vibrations Bending vibrations Fig. 9.3. Molecular vibrations caused by infrared radiation. Since different bonds andfunctional groups absorb at different wavelengths, an infraredspectrum is used to determine the structure of organic molecules. For example, carbon-carbon triple-bond is stronger than a carbon-carbon double bond and requires a shorter wavelength (greater energy) to stretch. The same considerations apply to carbon-oxygen and carbon-nitrogen bonds. C=C 2100-2200cm c=c 1620-1680cm ri C=0 1690-1750cm C—O 1050 1400an C=N 2210-2260cm"1 C—N 1050-1400cm1 Thus, from the positionofan absorption peak,one can identify the group that caused it.Fig. 9.4 shows the general areas in which various bonds absorb in the infrared. Wavelength (jam) 2 100 . I o 80 .1 I60 c CO i<0 iX 20 "T1"" N — H O— C 3.5 4 4.5 5 5.5 6 6.5 7 8 9 10 11 12 14 16 1" ' C =r C c3n h°HN C =N T ÿJ—1—i—i — C ~C :C~0 C N Fingerprint rQgioft P i ÿ T .4000 3500 3000 2500 2000 1800 1600 1400 1200 1000 800 600 Wavelength (cm ') Fig. 9.4. Area of absorption for various bonds in the infrared.
- 4. SPECTROSCOPY ÿ 175 (B> (C) Fig. 9.5. bend ÿC —H stretchOH J stretch CO stretch An infrared spectrum is usually studied in two sections : (1) FunctionalGroup Region.The area from 5000cm'1to 1300cm"1iscalledthe functional group region. The bands in this region are particularly useful in determining the type of functional groups present in the molecule. (2) Fingerprint Region.The area from 1300 cm"1to 667 cm"1iscalledthe fingerprint region. A peak-by-peak match ofan unknown spectrum with the spectrum of the suspected compound in this regioncan be used, muchlikea fingerprint, to confirm itsidentity.Table 9.1shows some characteristic infrared absorptionbands. Fig. 9.5 shows some examples ofinfrared spectra. Wave numbers in cm"1 5000 4000 3000 2500 2000 1500 1400 1200 1100 1000 900 800 700 100 s ......- TT ÿ Wave length in microns stretch Wave numbers in cm"1 Wave length in microns stretch C-H bends (isopropyl) bends Hstretch 5000 4000 Wave numbers in cm"1 1100 1000 900 bends KCHoNHo_I _— L -£ 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Some examples of IR spectra. (A) is Methanol. (B) is Butanone. (C) is Isobutylamine.
- 5. 176ÿ A TEXTBOOK OF ORGANIC CHEMISTRY Table 9.1 :SOME CHARACTERISTIC IR ABSORPTION BANDS Range incm'1 Bond(Remarks) 1050-1400 C-0 (inethers, alcohols, esters) 1050-1400 C-N (inamines) 1315-1475 C-H (inalkanes) 1340-1500 no2 (two peaks) 1450-1600 c=c (inaromatic rings :several peaks) 1620-1680 C=C (inalkenes) 1630-1690 C=0 (inamides) 1690-1750 C=0 aldehydes, ketones, esters) 1700-1725 c=o (incarboxylic acids) 1770-1820 c=o (inacidchlorides) 2100-2200 Cs=C 2210-2260 C=N 2500 S-H 2700-2800 C-H (ofaldehyde group) 2500-3000 O-H (of COOH group) 3000-3100 C-H (C is part ofaromatic ring) 3330 C-H (C is part of OC) 3020-3080 C-H (C is part of C=C) 2800-3000 C-H (inalkanes) 3300-3500 N-H (inamines,amides) 3200-3600 O-H (in H-bonded ROH) 3600-3650 O-H Summary of IRSpectroscopy (1)Absorption ofinfraredradiationcauses covalent bonds within the moleculeto be promoted from one vibrational energy level to a higher vibrational energy level. (2) Stronger bonds require greater energy to vibrate (stretch or bend). Therefore, such bonds absorb infrared radiation ofshorter wavelengths. (3) Different functional groups absorb infrared radiation at different wavelengths, and their presence or absence ina moleculecan be determined by examination ofan IR spectrum. (4) No two compounds have exactly identical infrared spectTa. ULTRAVIOLET-VISIBLE SPECTROSCOPY Inultraviolet-visible(UV-Vis)spectroscopy, the 200-750 nmregionofthe ultravioletspectrum is used. This includes both the visible region (400-750 nm) and near ultraviolet region (200-400 nm). Radiation of these wavelengths is sufficiently energetic to cause the promotion of loosely held electrons, such as nonbonding electrons or electrons involved in a 7i-bond to higher energy levels. For absorption inthis particular regionofultravioletspectrum, the moleculemust contain conjugated double bonds. Ifthe conjugation is extensive, the molecule will absorb inthe visible region. The ultraviolet-visiblespectrum is composedofonly a few broadbands ofabsorption(Fig.9.6). The wavelength ofmaximumabsorbance isreferredto as The following pointsshouldbekeptin mindwhile interpretinga UV-Visspectrum. (1) Nonconjugatedalkenesshow an intenseabsorption below200nmandistherefore inaccessible to most commonly usedUV spectrometers. Forexample,ethylenehas =171nm.This absorption comes from the light-inducedpromotionofa 7c-electronto the next higher energy level. I
- 6. SPECTROSCOPY. ÿ 177 .....V -I .. ' )iM|m IO' ÿ - / j --f- I 200 400 600 750 nm Fig. 9.6. A sample UV-Vis spectrum. (2) Nonconjugatedcarbonyl compounds have a very weak absorption band in the 200-300 nm region.This bandarises fromexcitationofone ofthe nonbondingelectrons(fromanunsharedpair)to the next higherenergy level.For example, O ii ch3—c—ch3 a0Acetone Cydohexanone 279 nm 291 nm (3) When a moleculecontains two or morenonconjugatedcarbon-carbondoublebonds,the UV spectrum isthat expectedofa simple alkene. However,when the doublebonds are conjugated, /™ax is shifted to longer wavelengths. For example, ch2=ch—ch2—ch2—ch=ch2 ch3ch=ch—ch=chch3 1,5-Hexadiene 2,4-Hexadiene 178 nm 227 nm (4) Conjugation of a carbon-carbon double bond and a carbonyl group shifts the Ama* of both groups to longer wavelengths. For example, (CH3)2C=CH—CH2CH3 180 nm O II (CH3)2CH—CH2—C—CH3 283 nm O (CH3)2C=CH—C—CH3 230 nm ( for C=C) 327 nm ( for C=0) (5) As the number ofdouble bonds inconjugationincreases, A,max also increases. For example, ch2=ch—ch=ch2 1,3-Butadiene Benzene 215 nm 257 nm Summary-ofUV-VisSpectroscopy (1) Absorptionofultraviolet-visibleradiation(200-700nm)causes electrons withinmolecules to be promoted from one energy level to a higher electronic energy level. (2) Ifan organic compound does not absorb UV-Vis radiation, it means that the compound does not contain conjugated double bonds. (3) Ifan organic compoundabsorbs UV-Visradiation, it means that the compoundcontains a carbonylgroup or conjugateddoublebonds.Forexample,conjugateddienes, carbonylcompounds, andaromatic compounds all absorb inthe UV-Visregion.
- 7. 178« atextbook of organic chemistry NUCLEAR MAGNETIC RESONANCE (NMR) SPECTROSCOPY Nuclear magnetic resonance spectroscopy involves absorption of electromagnetic radiation in the radio frequency region.Absorption ofradiowaves inthe presenceofmagnetic field isaccompanied by a special type of nuclear transition, and for this reason we call this type of spectroscopy nuclear magnetic resonance (NMR) spectroscopy. The nuclei ofcertainatoms behaveas ifthey are spinning charges. Any spinning charge creates a magnetic field and behaves as if it were a tiny bar magnet. Of the three nuclei most common in organic compounds ('H, l2C, and ,60)only the hydrogen nucleus proton behaves in this manner. When a proton inan organic molecule isplaced in a strong magnetic field, it can align with the field or against it. Inthe morestable low-energystate, itisalignedWith the magnetic field(Fig.9.7). If energy issupplied inthe form ofradiowavesofexactly the right frequency, radiation will be absorbed and the nucleus will "flip" and alignagainstthe appliedmagnetic field inthe higher energy state. For example,a proton inan appliedfieldof 14000 gauss requiresa frequency of60 millionhertz(cyclesper second) for this particular transition. When this exact frequency is applied, it is absorbed, and the absorption recorded on a chart paper. Inpractice, either the magnetic field can be held constant and the radio frequency varied, or more commonly, the radio frequency can be held constant and the magnetic field varied. Fig.9.8 shows the various components ofa NMR spectrometer. Positionof Signals ;ChemicalShifts. The mainpurpose ofNMR isnot to detect the presenceof protons in a molecule. It can distinguish between protons indifferent chemical environments within the molecule. Protons on benzene ring, or on a carbon bearing a chlorine, or on a carbon adjacent to a carbonyl group absorb radio frequency energies at different applied magnetic fields, and appear at different locations(chemicalshifts) on the recordingpaper. Also, thepositionofabsorption isrelatively constant for protons ina particular chemical or structural environment. Hence,the numberofsignals recordedon the NMR chartpaper indicates the number ofdifferent types ofprotons ina molecule. The position of the peak can give information about the molecular structure in the vicinity of the proton. High energy less stable state (b) Radio waves T Low energy more stable state (a) Fig. 9.7. Orientation of a spinning hydrogen nucleus in an applied magnetic field. (a) shows the nucleus in more stable low-energy state. (b) shows the nucleus in less stable high-energy state. Applying radiowaves flips the nucleus from the more stable 'aligned' state (a) to less stable 'nonaligned' state (b). To understandthe applicationofNMR,we should knowwhat arc EquivalentandNon-equivalent protons.Equivalent protons are protons inthe same moleculethat are chemically indistinguishable. In I
- 8. SPECTROSCOPY J 179 other words, equivalent protons are positioned instructurally and chemically equivalent areas inthe molecule.As an example, letus consider the following compounds. Sample tube (A) Magnet (B) Radio frequency input oscillator Detecto Radio Frequency Oscillator NMR spectrum Detector Powerful electromagnet Sample tube Fig. 9.8. Schematic diagrams of a NMR spectrometer. CI ch3—ch2—CI a b CH3—CH2—CH2—CI a b c n-Propyl chloride (3 Signals) CH -CH,CH- a" b c" Isopropyl chloride (2 Signals) Ethyl chloride (2 Signals) Equivalent protons are indicated by the same letter, non-equivalent protons by different letters. Noticethat w-propyl chloridecanbe easily distinguishedfrom its isomer isopropylchloride,by simply examining the NMR spectra of the two compounds, n-propyl chloridc gives three NMR signals and has three non-equivalent sets ofprotons,whereas isopropyl chloride gives two NMR signals and has two sets of equivalent protons and one non-equivalent proton.
- 9. 180". A TEXTBOOK OF ORGANIC CHEMISTRY Delta and Tail Scales.Nuclearmagneticresonancechartpaperisrectangularwith a linearscale of 8 (delta) unitsacross the bottom.Mostchart papers have scales from 5=0to 8= 8 or 9 ppm (partsper million).Toeverysampleasmallamount ofTetramethylsilane (TMS),(CH3)4Si,isaddedasareference (theTMS signal isat 8=0). All other signals are relativeto TMS. SometimesX (tau)scaleisusedwhere the TMS signal isat 10.0ppm. HenceT = 10—8. Table 9.2 lists some characteristic chemical shifts (fromTMS) ofdifferent types ofprotons. Table 9.2 : SOME TYPICAL NMR CHEMICAL SHIFTS OF PROTONS pe ofProton Type ofCompound 8 Value R-CH3 Alkane 0.8-1.2 Ar—CH3 Arene 2.2-2.5 c=c-ch3 Alkene 1.6-1.9 R-O-CH3 Ether 3.2-3.3 Ar—O-CH3 Ether 3.7-4.0 R-CO-CH3 Ketone 2.1-2.4 Ar—CO-CH3 Ketone 2.4-2.6 CH3-COOR Ester 1.9-2.2 R-COOCH3 Ester 3.0-3.9 CH,-N< Amine 2.2-2.6 R-CH2-R Alkane 1.1-1.5 R-CH2—Ar Arene 2.5-2.9 R-CH2-X Alkyl halide 2.7-4.5 r-ch2-no2 Nitroalkane 4.4-4.6 R CH2-OH Alcohol 3.4-4.0 Ar-CH2-OH Alcohol 4.0-5.0 R-CH2-COOH Acid 2.0-2.6 R3C-H Alkane 1.4-1.6 Ar-H . Arene 6.0-7.5 C=C—H Alkene 4.5-6.0 CsC-H .Alkyne 1.7-1.9 R-CHO Aldehyde 9.0-10.0 R-COOH Acid 10-12 R-OH Alcohol 2-6 Ar-OH Phenol 6-8 r-nh2 Amine 1-5 As anexample, letusconsider the NMR spectrum ofbenzylalcohol(Fig.9.9A). Noticethat there are four signals inthis spectrum. (1) The signal on the far right at 8= 0 is that ofthe TMS reference. (2) The signal at 8 = 7.3 is a single sharp peak due to the five chemically equivalent phenyl protons. (3) The signal at 8 =4.6 isa single sharp peak due to the two chemically equivalent methylene (-CH2-) protons. (4) The signal at 8=2.4 isa single sharp peak due to the hydroxy (-OH) proton. I
- 10. SPECTROSCOPY ÿ 181 Area = 5 Area = 2 TMS Reference 8.0 7.0 6.0 5.0 3.0 2.0 1.0 0 PPM4.0 Chemical Shifts / 5 (B) Area = 3 Area = 2 Area = 1 1.0 0 PPM8.0 7.0 6.0 5.0 4.0 3.0 2.0 Chemical Shifts / 8 Area = 6 "CH? Ares TMS Reference! 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0 PPM Chemical Shifts / 5 Fig. 9.9. Some examples of NMR spectra. (A) is Benzyl alcohol. (B) is Ethyl alcohol. (C) is Isopropylbenzene. Peak Area ;Integration.The relative areas under the various peaks ofa NMR spectrum are in proportion to the number of protons contributing to each signal. These areas can be electronically integrated by a NMR spectrometer. Comparison of the areas provides the ratio among the various kindsofprotons inthe molecule.Forexample,consider the NMRspectrum ofbenzylalcohol(Fig.9.9 A). The hydrogens inthe moleculeare ina I:2 :5 ratio,likethe correspondingareas inthe spectrum. I
- 11. 182 iA TEXTBOOK OFORGANIC CHEMISTRY Peak Splitting ;SplittingPattern.Hydrogensonadjacent carbons,eachwithadifferent chemical shift, can influencethe signal ofthe other. This influenceappears as peak splitting. We can generalise the phenomenon by saying that the number ofpeaks into which aparticularproton'ssignal is split, equals one more than the total number ofprotons on directly adjacent carbons. The split peaks are not ofequalheight. Fig. 9.10 shows the splitting pattern for various types ofnon-equivalent protons. II I H I H II I I I I —C— C— —C—c— —C—c—H ii ii ii H H H H H H / I / Doublet Doublet Triplet Doublet Quartet Doublet Fig. 9.10. Splitting pattern for various types of non-equivalent protons. Inthe NMR spectrum of ethyl alcohol (Fig. 9.9 B), notice that the ethyl group is indicated by a quartet and a triplet. In the NMR spectrum of isopropyl benzene (Fig. 9.9 C), notice that the isopropyl group is indicated by a heptet and a doublet. SummaryofNMRSpectroscopy (1) Absorption ofradiowaves inthe presenceofa magnetic fieldcauses nucleiwithin molecules to be promoted from one spin energy levelto a higher spin energy level. (2) The number ofsignals inthe NMRspectrum corresponds to the number ofdifferent types ofprotons inthe molecule. (3) The position (chemical shift) of each signal gives information about the structural environment of the protons. (4) The relative areas (Integration) under the signals givethe ratioofthe numbers ofeach type of proton inthe molecule. Ifthe molecular formula is known, the actual number of each type of proton can be determined. (5) The splittingpatternofeachsignal gives usthe numberofprotonsonneighboringcarbons. The number ofpeaks into which a signal is split is one more than the total number ofprotons on directly adjacent carbons. IMPORTANT TERMS USED IN NMR SPECTROSCOPY Chemicalshift :The separationbetween thepeak ofthe reference standard (TMS) andany other peak inan nmr spectrum. 8scale :An nmr scale inwhich the TMS peak isset at zero andpeaks to the left ofit (downfield) haveincreasinglypositivevalues, inparts per million(ppm). TMS :Abbreviation for tctramethylsilane, (CH3)4Si,the reference standard innmr spectrometry. Downfield shift :The protons of most organic compounds are less shielded than those of TMS and therefore require less energy for resonance to take place. The peaks appear therefore at lower fields, or downfieldof the TMS reference peak. Spin-spin coupling : The splitting of a signal into two or more peaks because of magnetic interactions between neighboring protons. (w +1) rule :A rulethat allowsus to predict intohow many smaller peaks a given signal issplit by neighboring protons. The rule states that a signal is split into (n+ 1) peaks, where n represents the number ofequivalent next-doorprotons.
- 12. SPECTROSCOPY ÿ 183 c I1 O JC C= ÿC N 1°» lo —H C =C 1 N— H II 3800 3400 3000 2600 2200 1800 1400 Wavenumber (cm*1) Group Assignments in Infrared Spectra C02H CHO ArH C=CH X—CH C=CH Tl*IS OCH COCK CH ch2 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0 Chemical shift, 8 (ppm) Higher magnetic field i >Proton more deshielded Group Assignments in NMR Spectra MASS SPECTROSCOPY Ina mass spectrometer, organic molecules are vapourised and bombarded with a beam of very high-energy electrons (Fig.9.11).The resultingcollisions impartconsiderable energyto the molecule, which inturn emit electrons to producepositively charged ions. These ions possess so much energy that they often fragment through various bond cleavages to produce new positively charged ions. ABC+ (Molecular ion) ABC electron > AB+ c+beam A+ BC+ The positive ions are accelerated toward a negatively charged plate by passing them through a magnetic field,which deflects the ions.The ionsoflightermass are deflected more thanheavier ions. Eachkindofionhasaparticularmassto charge (m!e)ratio.Formost ions producedinthe fragmentation of the molecule, the charge is +1so, that m/e usually represents the mass ofthe ion. Base Peak.The set ofionsproducedfrom amoleculecan be analysed since each has itsown ml e ratio,andproduces a signal whose intensity is due to the relativeabundace of that ion. The largest peakfound in a mass spectrum (that ofhighest intensity) is calledthe basepeak, and is given the numerical value of 100. The intensities of all other peaks are expressed relative to the height ofthe base peak.A mass spectrum ishighly characteristic ofa particular compound. I
- 13. 184" A TEXTBOOK OF ORGANIC CHEMISTRY Beam positive Negatively accelerating plates Sample=(o inlet xS Electron gun (B) Negatively charged accelerating plates Sample inlet Fig. 9.11. Schematic diagrams of a Mass spectrometer. Molecular Ion. The ionformedby removing one electronfrom theparent molecule is called molecular ion orparent ion. The molecular ionpeak is usually representedas NT.Itmay or may not be the peak of highest intensity. The molecular ion is the most important ion, since its mass is the Molecular Weight ofthe parent molecule. The use of mass spectroscopy is not limited to the determination of the molecular weight of compounds. Italso has great utilityinthe elucidationofthe structure ofa molecule.The fragmentation processes oforganic moleculesfollow certainpatterns inthat the fragments brokenoffarerelatedto bond strengths and the stability of the species formed. Since certain structural features inmolecule producedefinite characteristic fragmentation patterns, the identificationofthese fragments and their relative intensities ina mass spectrum can beused to determine the structure of organic compounds. The following examples illustrate how a mass spectrum can be used to determine the structure of organic molecules: Example 1. Let us consider the following isomers. CH3CH2CH2CH2CH2CH2CH2CH3 n-Octane ch3 CH3CH2CH2CHCH2CH2CH3 4-Methylheptane 77-Octane canbeeasily distinguishedfrom 4-methylheptane, since the methyl group branched at C-4inthe lattercompound, is easily brokenoff"as CHÿ fragment and will give a peak oflargeintensity at m/e= 15 Example 2. Consider the mass spectrum shown in Fig. 9.12. The fragmentation pattern and relativeabundance ofpeaks isas follows.A molecular weight of32,whichcorresponds to 02,isruled I
- 14. SPECTROSCOPY ÿ 185 out, since the only other possible fragment would be O with a peak ofrelatively high intensity at m/ e=b.Anm/epeak is present at a value of 16,but has a very low relative intensity,0.21. The peak at m/e= 15 isdue to a CHt fragment, and the overall spectrum indicates an elemental composition of CH40.Considerationofother peakspresent leads to the conclusionthat the moleculeproducingthis spectrum ismethylalcohol (CH3OH). 100; 3 40 m/e Relative abundance 12 0.32 13 0.72 14 2.4 15 1.3 16 0.21 17 1.0 28 6.3 29 64 30 3.8 31 100 32 66 33 0.98 34 0.14 Fig. 9.12. Mass spectrum of Methyl alcohol, CH3OH SummaryofMassSpectroscopy (1) Mass spectroscopy involves organic molecules being bombarded by a very high-energy electronbeam. (2) The peak ofhighest intensity in a mass spectrum is referred to as the base peak. (3) Fragmentationprocesses can produce numerous fragments, from which the structures of organic molecules can be deduced. (4) When one electron is removedfrom a molecule,a molecular ion isproduced.Them/evalue of the molecular ion peak is the molecular weight of the compound being investigated. The molecular weights obtained by mass spectroscopy arc extremely accurate. o Mass |Spectrometer Moleculefar,d Here
- 15. 186" A TEXTBOOK OF ORGANIC CHEMISTRY EXAMINATION QUESTIONS 1. (a) Explain why spectroscopic methods are increasingly used for structure determination of organic compounds. (b) What type of information one can obtain from : (/) IR spectrum ; (if) UV spectrum ;and (/'/'/) NMR spectrum. 2. What type of molecular transitions are brought about by absorption of : (a) Ultraviolet-visibleradiation (b) Infraredradiation (PanjabBSc, 2003 ;DelhiBSc. 2005) 3. Write notes on : (a) UV spectroscopy (Banaras BSc. 2001) (b) IR spectroscopy (MadrasBSc, 2003) (c) NMR spectroscopy (DelhiBSc, 2002 ;Jadavpur BSc. 2004) 4. Discuss the principle and applications of NMR spectroscopy in structure determination of organic compounds. (HSGaur BSc. 2004) 5. What ismeantby the term ChemicalShiftofa particularproton inNMR spectroscopy ? Draw the splitting patterns of protons in NMR spectrum of ethyl alcohol. 6. How will you distinguish between 1,3-pentadiene and 1,4-pcntadiene by UV spectroscopy ? Answer. 1,3-Pentadiene will have higher max than 1,4-pcntadiene. This is because 1,3-Pcntadiene is conjugated ;whereas 1,4-pentadiene is not. 7. How will you distinguish between benzene and anthracene by UV spectroscopy ? (Bharathidasan BSc. 2004) Answer. Anthracene will have higher max than benzene. This is because anthracene is more conjugated than benzene. 8. A chemist prepared the two isomeric ketones (A ) and (B). placed them in separate flasks, but forgot to label them. How could you differenciate the two by UV spectroscopy O O II II ch3ch2ch=chcch3 ch3ch=chch2cch3 (A) (B) Answer. In the UV spectra, (.4),a conjugated system, would show Xmax at higher wavelengths than (B),a nonconjugatedcompound. 9. Two isomeric dienes (A) and (B) having the molecular formula CsHg absorb at 223 nm and 178 nm respectively. Write the structures of the two isomers. Answer. (A) is CH2=CH-CH=CH-CH3 ; 1,3-Pentadiene (B) is CH2=CH-CH2—CH=CH2 ; 1,4-Pentadiene 10. Arrange the following compounds inthe increasingorder of their UVabsorption maxima : (a) Ethylene (b) Naphthalene (c) Anthracene (d) 1,3-Butadiene Answer, (a) < (d) < (c) < (b) 11. How do you identify the following functional groups by IR spectroscopy : (a) -OH (b) -NH2 (c) -CN (d) >C=0 12. Discuss the principle of IR spectroscopy. How will you distinguish between the following pairs of compounds on the basis of IR spectroscopy : (a) Ethyl alcohol anddiethyl ether (b) Acetic acid andethyl acetate Answer, (a) Ethyl alcohol shows a strong/broad absorption at 3200-3500 cm-1(due to O-Hbond) while diethyl ether does not ; and (ft) Acetic acid shows a strong/broad absorption at 2500-3500 cm"1 (due to O-H bond) while ethyl acetate docs not. 13. How would you distinguish between the following pairs of compounds by IR spectroscopy ? (a) CH3CH2CH2N(CH3)2 and CH3CH2CH2NH2 (b) CH3CH2CH2COOH and CH3CH2COOCH3 (c) CH,CH2COCH3 and CH3CH2COOCH3 I
- 16. r SPECTROSCOPY J 187 Answer, (a) CH3CH,CH:NH2 shows N-H absorption at 3300-3500 cm"1 while CH3CH2CHjN(CH,)2 does not. (b) CHjCH2CH2COOHshows O-Habsorptionat 2500-3500cm 1 whileCH3CH2CH2COOCH3 does not. (c) CH3CH2COOCH3 shows C-0 absorption at 1050-1350 cm1while CH3CH2COCH3 does not. 14. Two compounds, (A) and (B), have the same molecular formula C2H60. They have different IR spectra. Compound (A) shows a strong/broad absorption at 3400 cm"1, while compound (B) does not. Suggest formulas for (A)and(B)which account for the difference. Answer. (A) is ethyl alcohol (CH3CH2OH) ;(B) is dimethyl ether (CH3OCH3). The strong absorption at 3400 cm"1 in(A) is due to the O-Hbond. 15. Draw the structure of a compound with each of the following molecular formulas that will show only one peak in its NMR spectrum : (a) C3H$C12 (b) C5HI2 (c) CÿO (d) C4H6 Answer. Possible structures are : CI ch3 (a) CH3-C-CH3 (b) CH3-C-CH3 CI CH3 (c) CH3-O-CH3 (d) CH3CSCCH3 16. How many NMR signals do you expect from eachofthe following compounds. Indicatealso the splitting pattern of the various signals. (a) CH3OCH3 (*>) CH3OCH2CH3 (c) CH3CH2OH Answer, (a) One signal(singlet) ; (b)Three signals (singlet,quartet,triplet) ;and(c)Twosignals (singlet, quartet). 17. How many signals (ignoring the splitting patterns) would you see in the NMR spectra of the following compounds ? (Saugar BSc, 2002) (a) Butanone (b) Cyclobutane (c) ÿ-xylene (d) 2-Propanol Answer, (a) Three signals ;(b) One signal ;(c) Two signals ;and (d) Three signals. 18. Predict the signal pattern of the CH3 protons in the NMR spectra of the following compounds. O II (a) CH3CHBr2 (b) CH3—C-OH OH (c) CHaCHjBr (d) CH3-CH-CH3 (AllahabadBSc.2003) Answer. Apply the (w + 1) rule, (a) The CH3 group has one neighboringproton and would therefore be a doublet ;(b)The CH3group hasnoneighboringproton,andwould therefore beasinglet ;(c)The CH3group has two neighboringequivalent protons andwould thereforebeatriplet ;and(d)The two CH3 groups are equivalent and haveone neighboringproton. The CH3signal would therefore be a doublet. 19. The NMR spectrum of compound (A) C2H60, shows one signal only, a singlet. Deduce the structure of (A). {CalicutBSc, 2004) Answer. (A) is CH3-0-CH3 20. The NMR spectrum of compound {A), C5H]2, gives only one signal, a singlet. What is the structure of (ÿ4)? Answer. The molecular formula, C5H12, indicates that (/*) is an alkane. The single signal in the NMR spectrum indicatesthat all hydrogensare equivalent. The only possibleC5H12 isomerthat fitsthe data is(CH3)4C (2,2-Dimethylpropane). 21. The UV spectrum for a compound with formula C3H60 shows a weak absorption band at 280 nm. The NMR spectrum shows only one signal, a singlet. What is the structure of the compound ? Answer. The weak UV absorption at 280 nm is characteristic ofa carbonyl group (C=0), leavingC2H6 for theremainder ofthe original formula. This suggests two CH3 groups. The structure ofacetone (CH3COCH3) fits all the data.
- 17. v 188!A TEXTBOOK OFORGANIC CHEMISTRY 22. The NMR spectrum of a compound C4HqBr consists ofa single sharp line. What is the structure of the compound ? Answer. The compound is /er/-Butyl bromide, (CH3)3 CBr. 23. Suggest the structure of a compound each with the following NMR spectral features : (a) An alcohol with two NMR peaks (6) A compound C5Hj0 with a single NMR peak (c) A compound C4H6 with a single NMRpeak. Answer, (a) Methylalcohol ; (b) Cyclopentane : (c) 2-Butyne 24. How would you distinguish between the following pairs of compounds byNMR spectroscopy ? O O II II (a) CH3-C-CH3 and CH3CH2-C-H O 0 II II (b) CH3—C—CH3 and CH3—C-OCH3 (PunjabBSc. 2005) Answer, (a) CH3COCH3 will give only one signal, while CH3CH2CHO will give three signals; and (b) CH3COCH3will give only one signal, while CH3COOCH3will give two signals. 25. How will you distinguish between the three dibromobenzenes by their NMR spectra. (Visva BhartiBSc, 2003) Answer. o-Dibromobenzene will show two peaks ;w-dibromobenzene will show three peaks ; and p-dibromobenzenewill show only one peak. Br Br V a Br Qr*b a c b o-Dibromobenzene m-Dibromobenzene p-Dibromobenzene (two peaks) (three peaks) (one peak) 1:1 1:2:1 26. Give a structure consistent with the following NMR data : Molecularformula =C3HsCl3 (a) Singletat 82.20, 3H (b) Singlet at 64.02, 2H b a Answer. The compound is CH2C1—CCI2—CH3 27. Give a structure consistent with the following NMR data : Molecular formula = Ci0H14 (a) Singlet at 5 1.30,9H (A) Singlet at 5 7.28,5H Answer. The compound is : fert-Butylbenzene CH3ÿ 28. Suggest a structure consistent with the following NMR data :Molecular formula = CÿHÿ (a) Singlet at 86.78, 3// (A) Singlet at 82.25, 9H (MysoreBSc.2004) Answer. The compound is : Mesitylene CH) a
- 18. r Alkanes <4- «* C C C j5,il.K '*!ÿ 1 loÿ 3UTANE mFUEL ; A: Butaneisusedasafuelinlighters. Itisalsousedinsamecamping stoves. lkanes are the simplest organic compounds made of carbon and hydrogen only. They have the general formula C„H2*+2where n= 1,2,3etc. The first three members ofthis class can be represented as : H H H H—C- I H -H H-C I H -C—H Methane H Ethane H H H I I I H—C—C—C—H I I I H H H Propane The carbon atoms intheir moleculesare bondedto each other by single covalent bonds. Each carbon is again bonded to enough hydrogen atoms to give maximum covalence of 4. Since the carbon skeletonofalkanes isfully 'saturated' with hydrogens,they are also calledSaturated Hydrocarbons. Alkanes containstrongC—C andC—Hcovalent bonds.Therefore, this class ofhydrocarbons are relatively chemically inert. Hencethey are sometimes referredto as Paraffins(Latin,parumaffinis= littleaffinity). STRUCTURE Let us consider methane (CH4) and ethane (CH3—CH3) for illustrating the orbital make up of alkanes. Inmethane, carbon forms single bonds with four hydrogen atoms. Since the carbon atom is attached to four other atoms, it uses spl hybrid orbitals to form these bonds. Each C-H bond is the resultofthe overlap ofansp3 orbitalfrom carbonand ans orbital from hydrogen(Fig. 10.1).All C—H bonds are a bonds.
