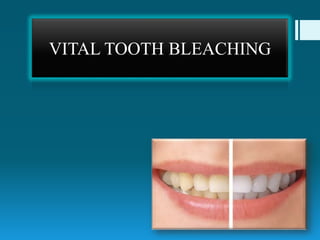
Vital tooth bleaching
- 2. Contents: Introduction History Bleaching agent Classification of Bleaching technique. Vital bleaching technique Effect of vital bleaching on tooth structure Effect of vital bleaching on tetracycline stain Effect of vital bleaching on Fluorosis stain Effect of vital bleaching on restorative material Conclusion References
- 3. • Tooth discoloration is a common problem leading patients to seek esthetic treatment. • The causes of tooth staining must be carefully assessed for better prediction of the rate and the degree to which bleaching will improve tooth color, since some stains are more responsive to the process than others. Jordan, R.E., Boksman, L.Contin. Educ. Dent.1984;5:803–807.
- 5. Extrinsic stains • Plaque, chromogenic bacteria, surface protein denaturation • Mouthwashes (e.g., chlorhexidine) • Beverages (tea, coffee, redwine, cola) • Foods (curry, cooking oils and fried foods, foods with colorings, berries, beetroot) • Antibiotics (erythromycin, amoxicillins • Iron supplements
- 6. • These stains are localized mainly in the pellicle and are either generated by the reaction between sugars and amino acids or acquired from the retention of exogenous chromophores in the pellicle. • The reaction between sugars and amino acids is called the ‘‘Millard reaction’’ or the ‘‘non-enzymatic browning reaction’’ and includes chemical rearrangements and reactions between sugars and amino acids. Viscio, D.et al.Compend. Contin. Educ.Dent. Suppl. 2000. 28, S36– S43, quiz S49.
- 7. Intrinsic stains Pre-eruptive Disease: • Hematologic diseases • Diseases of enamel and dentin Medication: • Tetracycline stains • Fluorosis stains Post eruptive • Trauma intrapulpal hemorrhage pulp necrosis • Primary and secondary caries and erosion, buccal and palatal . • Dental restorative materials endodontic materials. • Aging
- 8. The lightening of color of the tooth through the application of a chemical agent to oxidize the organic pigmentation in the tooth. Bleaching — the use of a chemical agent, sometimes in combination with heat, to remove tooth discolorations. Extracoronal bleaching (external bleaching) — the use of a chemical agent on the outside of a tooth to remove discoloration from tooth structures; most frequently used agents are hydrogen peroxide and urea (Carbamide) peroxide. Bleaching: AAE Glossary of Endodontic Terms 2012 Grossman 12th edi
- 9. • Vital teeth were also bleached as early as 1868, by means of oxalic acid or Pyrozone and later with hydrogen peroxide. • In Fisher1911, the use of concentrated hydrogen peroxide with a heating instrument or a light source for tooth bleaching. . Alqahtani MQ et al. Saudi Dent J. 2014 Apr; 26(2): 33–46 History:
- 10. • Furthermore, in the late 1960s, a successful home-bleaching technique was established when Dr. Bill Klusmier, an orthodontist, instructed his patients to use an “over-the- counter” oral antiseptic, which contained 10% carbamide peroxide delivered via a custom-fitting mouth tray at night. • Dr. Klusmier found that this treatment not only improved gingival health but also whitened teeth. . Alqahtani MQ et al. Saudi Dent J. 2014 Apr; 26(2): 33–46
- 11. • Subsequently, Proxigel (a mixture of 10% carbamide peroxide, water, glycerin, and carbopol) was marketed. • Haywood and Heymann (1989) described a home-bleaching technique in their article, “Nightguard vital bleaching”, and an at-home bleaching product “White and Brite™” was introduced. . Alqahtani MQ et al. Saudi Dent J. 2014 Apr; 26(2): 33–46
- 12. • The “over-the-counter” (OTC) bleaching agents were first launched in the United States in the 1990s, containing lower concentrations of hydrogen peroxide or carbamide peroxide and sold directly to consumers for home use. • Finally, the current in-office bleaching technique typically uses different concentrations of hydrogen peroxide, between 15% and 40%, with or without light and in the presence of rubber dam isolation. . Alqahtani MQ et al. Saudi Dent J. 2014 Apr; 26(2): 33–46
- 13. Bleaching agents
- 14. Properties of the ideal whitening agent: • Easy to apply to the teeth for maximum patient compliance. • Be nonacidic (have a neutral pH). • Lighten the teeth successfully and efficiently. • Remain in contact with oral tissues for short periods. • Have an adjustable peroxide concentration. • Be nonirritating. • Not dehydrate the oral tissues or teeth. • Not cause damage to the teeth.
- 15. Hydrogen Peroxide: It is clear, colorless, odorless liquid, stored in amber colored bottles. Various concentration of this agent are available but 30- 35% stabilized solution of H2O2(superoxol) are most common. It is stored in refrigerated container where it retains sufficient potency for appro 3-4 months.
- 16. Carbamide Peroxide: It is also known as urea hydrogen peroxide, carbamyl peroxide,perhydrol urea (3-45%) Carbamide peroxide (CH6N2O3) in a 10% gel formulation is the most commonly used home whitening material. The material is normally supplied in a syringe for ease of application, although some products are supplied in blister packs.
- 17. CP breaks down to a 3.6% solution of hydrogen peroxide (H2O2) and a 6.4% solution of urea (CH4N2O).
- 18. • The 15% carbamide peroxide solution (CPS) yields 5.4% hydrogen peroxide, and the 20% solution yields 7% hydrogen peroxide. • A 35% carbamide peroxide gel is available as Quickstart (DenMat, Lompoc, CA) and 45% gel as Opalescence Quick (Ultradent Products, South Jordan, UT).
- 19. UREA: • Stabilize the hydrogen peroxide. • Elevate the pH of the solution. • Enhance other desirable qualities, such as anticariogenic effects, saliva stimulation, and wound healing properties
- 20. THICKENING AGENTS Carbopol (carboxypolymethylene) • Carbopol is a polyacrylic acid polymer and its concentrations range from 0.5–1.5%. • Slow release of oxygen • enhances the viscosity of the whitening material. • Its thixotropic nature allows better retention of the slow- releasing gel in the tray and improves adherence to the tooth.
- 21. VEHICLE: GLYCERIN: • Carbamide peroxide is formulated with a glycerin base, which enhances the viscosity of the preparation and ease of manipulation.
- 22. SURFACTANT AND PIGMENT DISPERSANTS: • The surfactant functions as a surface wetting agent, which allows the hydrogen peroxide to diffuse across the gel– tooth boundary. • Gels with surfactant or pigment dispersants may be more effective than those without them
- 23. PRESERVATIVES • Methyl, propyl paraben, and sodium benzoate are commonly used as preservative substances. • They have the ability to prevent bacterial growth in whitening materials. • Many of the gels contain a preservative such as citroxain, phosphoric acids, citric acid, or sodium stannate. • These preservatives sequestrate transitional metals such as iron, copper, and magnesium, which accelerate the breakdown of hydrogen peroxide
- 24. FLAVORING AGENT: • Flavorings are used in whitening materials to improve the taste of the material, add to the selection of whitening agents, and improve acceptability of the product to the patient (e.g., melon and mint).
- 25. Mechanism of action of bleaching agent The bleaching process is based on oxidation of bleaching material. The oxidation-reduction reaction that takes place in bleaching process is known as REDOX REACTION. In this process the oxidizing agent has a free radical with unpaired electrons, which it gives up, becoming reduced. The reducing agent (i.e. the substance being bleached) accepts the electrons and becomes oxidized.
- 26. The H2O2 flow freely through the enamel and dentin owing to the porosity and permeability of these structures. The free movement is caused by the relatively low molecular weight of the peroxide molecule and the penetrating nature of the oxygen and superoxide radicals. The free radicals are produced have unpaired electron; they are unstable and hence will attack most other organic molecule to achieve stability.
- 27. This results in reduction of absorption energy of organic molecules in the tooth enamel, releasing the color thus creating a successful whitening action.
- 29. Vital Bleaching Techniques OTCIn office Home /Night- guard Bleaching
- 31. • Home whitening is a simple technique whereby, after an initial consultation with the dentist, a mouthguard or tray is made for the patient to whiten the teeth at home. Terminology: 1) Matrix bleaching. 2) Dentist-assisted or dentist-prescribed home- applied whitening. 3) Dentist-supervised at-home whitening. 4) At-home whitening.
- 32. 1. It is simple and fast for patients to use 2. It is cost-effective. 3. Patients can whiten their teeth at their convenience, according to their personal schedule. 4. Patients can see the results relatively quickly. Advantages 1. The color change is dependent on the amount of time that the trays are worn. 2. The system may be open to abuse by use of excessive amounts of whitening agent for too many hours per day . 3. It is difficult for patients who gag easily to tolerate the whitening trays in the mouth. Disadvantages
- 33. Indications • Mild generalized staining • Age-yellowing discoloration. • Mild tetracycline staining. • Very mild fluorosis (brown or white) • Acquired superficial staining. • Stains from smoking tobacco • Absorptive and penetration stains (tea and coffee) • Young patients with an inherited gray or yellow hue to the teeth
- 34. Contraindications: • Severe tetracycline staining, pitting hypoplasia, fluorosis stain. • Teeth with tooth surface loss from attrition, abrasion, and severe erosion. • Teeth exhibiting extreme sensitivity to heat, cold, touch, and sweetness. • Teeth with deep and surface cracks and fracture lines. • Teeth with pathology such as a periapical radiolucency.
- 35. Procedures: 1. Initial Consultation 2. Clinical Examination Of All Teeth 3. Planning The Treatment 4. Impression Taking 5. Tray fabrication
- 36. • The advantages and disadvantages of tooth whitening. • Any side effects that may be experienced (such as sensitivity, gingival irritation. • The risks and benefits of the procedures; the patient’s informed consent should be obtained. • The duration of treatment. • Further esthetic treatment that may be needed. Initial Consultation:
- 37. • A comprehensive examination should be performed to assess the soft tissues, mucosae, teeth, gingivae, and oral health status of the patient. • Check the integrity of the existing restorations. • Check recent radiographs for dental disease and periapical radiolucencies. • The size and vitality of the pulps of the teeth can be assessed on radiographs to predict sensitivity levels. Clinical Examination Of All Teeth
- 38. • Before treatment is begun, discuss with the patient the possible shade lightening that can be achieved. • Shade taking can be done via the normal methods—that is, using the porcelain shade guide or the shade guide supplied with the whitening kit. Preexisting Shade Evaluation
- 39. • It is normally advisable to whiten only one arch at a time so that the patient has the opportunity for a comparison. • Photographs with the shade tab that currently matches the teeth are taken. • It is also essential to discuss with patients that their existing composite restorations may not match after whitening and that it may be necessary to replace these composites with lighter ones after the whitening procedures. Planning The Treatment:
- 40. • Excellent impressions reproducing the surfaces of the upper and lower teeth should be taken so that whitening trays can be made. Impression Taking
- 41. • Be strong enough to avoid damage by the patient during wear. • Be easy to fit and easy to remove after a treatment session. • Be made from a material that is bioinert . • Be thin enough to be well tolerated in the mouth. • Be smooth and well polished so that there are no rough edges. • Fit comfortably and passively and not feel too tight in places. Tray making:
- 42. • Full vestibule upper or lower trays. • Trays with reservoirs • Trays with no reservoirs. • Trays with or without windows. Tray Design Features
- 43. The vacuum tray-forming machine. The separator is placed directly onto plaster teeth. The separator is air dried onto the plaster teeth. The plastic tray material. Tray Fabrication:
- 44. Remove the tray from the model The model is placed into the vacuum tray-forming machine. Securing tightly onto the vacuum tray-forming machine. Allowing the heated material to cool down.
- 45. The excess is removed . The fine trimming and the scalloping Final trimming of the tray on the model.
- 46. The tray is smoothed with a polishing wheel. The trays are scalloped lingually and buccally. Completed tray positioned in the mouth.
- 47. Loading Tray and placement of tray: A syringe loading gel is placed Seats the tray and compresses to ensure that the gel is placed properly. A blister pack application.
- 49. Gingival irritation. Patients may complain of painful gums after a few days of wearing the trays. It is important to check that the tray is fitting correctly and not impinging on the gingivae. The trays may need to be adjusted, trimmed back, polished.
- 50. Soft tissue irritation. Some patients develop soft tissue irritation, which may be from overwearing the trays or applying too much whitening agent to the trays.
- 51. Tooth thermal sensitivity. This is the most common side effect and normally occurs after about 2 or 3 days of wearing the trays. Desensitizing toothpaste can be used 2 weeks before whitening, by brushing the teeth with the desensitizing toothpaste or into the whitening tray or directly onto the sensitive parts of the teeth.
- 52. Tooth thermal sensitivity. Neutral sodium fluoride gel can be used for desensitizing; applied directly with a brush onto the teeth or it can be placed into the whitening tray . Proprietary agents containing potassium nitrate can be applied directly into the tray. The tray can be worn overnight.
- 54. Power bleaching or professional in-office bleaching is a term used to describe the treatment of discolored dentition with high-concentration oxidizing agents by the dentist chairside. Terminology: Chairside bleaching Laser bleaching Dentist assisted bleaching
- 55. 35% H2O2 liquid/gel 35% carbamide peroxide Dual activated bleaching system Materials available:
- 56. 1. It is simple and effective for dentist to use . 2. The treatment is dentist operated. Advantages 1. Expensive 2. Bleaching agent used are stronger and caustic in concentration Disadvantages
- 57. Power Bleaching Techniques Heated instrument Dual Activated power bleaching Compressive power bleaching Light sources Warming the bleaching agent
- 58. Miara (2000) suggested that power bleaching could be more effective by compressing the bleaching material on the tooth. Use of 35% H2O2 is recommended The benefit of this technique ; it influences penetration of oxygen ions into tooth enamel. Compressive power bleaching:
- 59. The Hi-lite in office bleaching system is formulated both light and chemical activation. It contains: Ferrous sulphate:-chemical activator ,completes procedure in 7-9 mins. Manganese sulphate:- light activator, fasten treatment 2-4 min Dual Activated power bleaching:
- 60. Uses H2O2 in high concentration 19-35% It has blue-green indicator dye which starts off blue and as it becomes deactivated it is changes to white. Dual Activated power bleaching:
- 61. Rubber dam isolation Guaze soaked with 35% H2O2 liquid placed on tooth. Heating instrument is applied for period of 1-3 mins to enhance effect of bleaching. Power bleaching using heat instrument:
- 62. The use of light to supplement the bleaching process in dentistry was reported as early as 1918 (Abbot 1918). Heat lamps (19th century–1980). Early bleaching lamps made use of an incandescent or photographic floodlight This type of light source produced a continuous spectrum with high infrared emission, which supplied a source of indirect heat. Power bleaching using Bleaching light:
- 63. Rubber dam isolation Guaze soaked with 35% H2O2 liquid placed on tooth left on tooth for 30 mins with light set 30 cm away from teeth. It is repeated at one/two week interval for 3-5 appointments.
- 64. • vital teeth, temperatures were recommended in a range of 46°C to 60°C (115°F to 140°F). • For nonvital teeth temperatures as high as 71°C (160°F) were recommended • The risk of increasing the pulpal temperature beyond the critical threshold of 5.5°C, at which irreversible pulpal damage can occur, is a concern with any system that raises the temperature of vital teeth. . • The use of heating lamps has fallen out of favor for vital teeth and may be considered obsolete by today’s standards. Zach and Cohen 1965, Baik et al. 2001
- 65. Halogen lamps (1980s–2000): • The halogen gas causes evaporated tungsten to redeposit on the filament, improving the filament life and allowing a higher color temperature than the standard incandescent lamp.
- 66. • Liang S et al. evaluate the effect of halogen light irradiation on in-office bleaching, in terms of HP concentrations, tooth whitening effects, colour stability, and temperature variations in bleaching agents and pulp chambers. (1) In-office bleaching system, with or without light irradiation, was effective for tooth whitening; (2) The involvement of halogen light could enhance the immediate whitening effect but have little influence on the long-term whitening effect over the four-week observation period. Liang S et al. Australian Dental Journal 2012; 57: 1–7
- 67. High-intensity discharge (HID) lamps (1990s–current). • High-powered lamps that produce light by ionizing noble gases (xenon, krypton) or metal halides between two electrodes.
- 68. • HID lamps may properly be referred to as metal halide lights and are often referred to as “plasma arc lights” • These lamps are typically wide-spectrum lamps using band pass filters to narrow the emission primarily to the short ultraviolet to blue light (380–500 nm)
- 69. • Nakamura, et al. (2001) and Kashima-Tanaka, et al. (2003) reported that the PAC significantly changed the tooth color. • whereas Hein, et al. (2003) and Lima, et al. (2009) demonstrated that the PAC did not effectively activate the bleaching gels Nakamura T et al. J Oral Rehab. 2001;28:1080-4. Kashima-Tanaka M, et al J Endod. 2003;29:141-3 Lima DA, et al J Prosthodont. 2009;18:249-54 Hein DK, et al. Compend Contin Educ Dent. 2003;24:340-52
- 70. Light-emitting diode (LED) lamps (2000–current). • Solid-state, semiconducting energy sources that supply near-monochromatic light. • An led bleaching light system is dependent less on heat and more on the wavelength-specific photochemistry of the bleaching formula and possible energy absorption of the natural tooth chromogens contributing to bleaching effect
- 71. • Kossatz (2011) reported a larger difference in bleaching with a light-emitting diode than without it (on 35% HP gel), with a shade guide value. • However, tooth sensitivity was higher for the LED treated group after 24 hours of treatment. Kossatz S, et al. Oper Dent. 2011 May-Jun; 36(3):251-7
- 72. • Yazici AR et al measured the temperature increase in the pulp chamber of extracted teeth produced by the Zoom!™ • Zoom!™ light either used with or without bleaching gel did not show a significant increase in the intrapulpal temperature of teeth when used for the recommended exposure time. Yazici AR, J Contemp Dent Pract 2007 May;(8)4:019-026
- 73. Rubber dam isolation 35% carbamide peroxide gel heated to 80°C and applied directly onto tooth. Power bleaching using heated bleaching gel :
- 74. • In preparation for bleach application, tooth plaque or superficial stain is removed using prophylaxis paste or pumice of flour paste in a rubber polishing cup Prophylaxis:
- 75. Initial Shade • Document the pre bleaching shade using shade guide. Light-protective Glasses And Light Guides • When using a supplemental light device, it is essential to provide the patient with light-protective eyewear to filter harmful radiation. • It has been shown that many lamps exceed standards set for eye exposure to direct blue light Bruzell et al. 2009.
- 76. LIP PROTECTION AND CHEEK RETRACTION A barrier cream or oil is applied to the lips before insertion of the cheek and lip retractors
- 77. GINGIVAL ISOLATION Resin barrier material is scalloped from papilla to papilla. Barrier seal is completed and ready for bleach application.
- 78. ACTIVATION OF BLEACH, IF REQUIRED: • A syringe-to-syringe mixing product will require the user to attach the syringe with the bleaching agent to a second syringe with the activator.
- 79. APPLICATION AND REAPPLICATION OF BLEACH • The bleach material is applied to the teeth in a layer 1–2 mm thick, generally for 15–20 minutes per application with three or four applications per session.
- 80. APPLICATION AND REAPPLICATION OF BLEACH • To avoid bleach splatter and dislodgement of the barrier material, surgical suction is used to remove the bleach between repeat applications of fresh material. REMOVAL OF BARRIER • The resin barrier is ready for removal and typically can be lifted in one piece by grabbing hold of the attached cotton rolls or dislodged using an explorer instrument
- 81. POSTBLEACHING SHADE • The post-treatment shade is taken to document the color change for the patient record and to demonstrate to the patient the immediate outcome.
- 82. POST-BLEACHING INSTRUCTIONS: • Post-bleaching instructions may include a recommendation to wait a minimum of 6 hours after in- office bleaching before drinking any chromogenic drinks such as coffee or grape juice. (Ontiveros et al. 2008). • To maintain the bleaching results, the patient can be provided a custom at-home bleach tray for periodic maintenance or for continued bleaching using the combination technique.
- 83. • Hein et al., 2003 reported no difference in the whitening effect of bleaching gels [25%-35% (HP)] with or without three different lights (LumaArch, Optilux 500, and Zoom!). • They concluded that the chemicals added to the bleaching gels acted as catalysts in the whitening process and were solely responsible for activation, where as the lights had no influence. • Hahn et al., 2013could not find an improvement in tooth whitening as a result of LED or laser light treatments, when evaluating the colour stability of bleaching with 38% hydrogen peroxide using four different methods: activation with halogen, LED, laser or chemical activation
- 84. • Ferrarazi et al., concluded that LED lamps are effective, safe and inexpensive to activate the hydrogen peroxide. • Dominguez et al., 2011 reported that only the diode laser, halogen lamp and LED lamp showed significant colour changes on three different 35% hydrogen peroxide whiteners. • It was concluded that the light source is more important than the bleaching agent in the whitening process. • He et al., 2012 reported that a light-activated system produced better immediate bleaching effects than a non- light system with lower concentrations of hydrogen peroxide.
- 86. The role of laser in teeth whitening is to accelerate the activation of hydrogen peroxide (H2O2) in whitening gels which typically contain 30% to 35% H2O2 concentration. In reaction to the absorption of photon, the hydrogen peroxide breaks down into particle of water and radical of oxygen.
- 87. • The free radical oxygen chemically reduces larger organic-pigmented molecules (the chromophores) in the enamel matrix into smaller, less pigmented constituents by rapid oxidation. • These compounds that originally have double bonds and long carbon chains are subsequently reduced to smaller carbon chains and hydroxyl groups, which eliminate discoloration.
- 88. Types of lasers: • Carbon dioxide • Argon (488nm) • Neodymium:yttrium-aluminium-garnet (Nd:YAG) • Erbium-chromium:yttrium-scandium- gallium-gamet (ErCr:YSGG)
- 89. 1. Faster due to higher concentration of active ingredient. Advantages 1. Expensive 2. Time consuming Disadvantages
- 90. Yarborough concept of bleaching: According to the concept ,mixture of 50% H2O2 in sodium perborate powder base. Argon laser energy is used to remove deep colored stains followed by CO2 laser that is absorbed rapidly by bleaching paste. The teeth are then cleaned, followed by final coating of fluoride gel.
- 91. Mathews et al (2014) Compare the clinical efficacy of an in-office Bleaching system using a 810 nm diode laser and conventional in-office bleaching system. Diode laser 810 nm has proven effective. Reza et al (2017) Compared the efficacy of power bleaching and laser bleaching technique and diode laser as an activator in their tooth whitening capacity. Both laser-assisted and power bleaching techniques were capable of altering tooth color change, but laser bleaching was deemed a more efficient technique in this regard.
- 92. OTC technique:
- 93. Bleaching tooth paste and mouthwash: OTC products are a low-cost alternative for white discolored teeth without dentist supervision. These products generally contain lower levels of a whitening agent and are self-applied to teeth by means of gum shields, strips, paint-on brushes, toothpastes, and mouthwash products.. They commonly require two daily applications for up to 2 weeks
- 94. • Trayless bleaching system. • Delivery system is a thin strip precoated with adhesive 5.3% H2O2 gel H2O2 strips:
- 95. • The backing is peeled off and the strip placed directly to the facial/buccal surfaces. • Each strip is worn for 30 minutes, removed and discarded and procedure takes place twice a day for 14 days.
- 96. ListerineWhitening mouthwash Water, alcohol (8%), hydrogen peroxide, tetrapotassium pyrophosphate, pentasodium triphosphate, citric acid, poloxamer 407, flavor, sodium saccharin, and sucralose Opalescence PF 10% Glycerin, water, xylitol, carbamide peroxide, flavor, carbomer, PEG-300, sodium hydroxide, potassium nitrate, EDTA, and sodium fluoride Crest 3DWhite Multi- Care whitening mouthwash Water, 1.5% hydrogen peroxide, propylene glycol, sodium hexametaphosphate, poloxamer 407, sodium citrate, flavor, sodium saccharin, and citric acid Details of mouthwashes products and bleaching gel
- 97. N. Sharif etal (2000) Measured the chemical stain removal properties of a range of whitening toothpaste products Only a small number of the whitening toothpaste products have good chemical stain removal potential. Karadas etal (2015) Analyze the efficacy of mouthwashes containing hydrogen peroxide compared with 10% carbamide Peroxide (CP) gel The color change achieved with the mouthwashes was significantly lower than that achieved with the 10% CP at-home bleaching gel.
- 98. Effect of bleaching on tooth structures:
- 99. Effects On Enamel: Smidt et al. (2011), evaluated the morphologic, mechanical, and chemical effects of 16%, 15% carbamide peroxide bleaching agents on human enamel in situ and they found that enamel surfaces showed no mechanical, morphologic, or chemical changes after bleaching. Smidt et al. (2011), Quintessence Int. 2011;42:407–412
- 100. • Cadenaro et al. (2010) conducted an in vivo study to test the effect of a hydrogen peroxide in-office whitening agent on enamel and demonstrated that the application of a 38% hydrogen peroxide in-office whitening agent did not change enamel surface roughness, even after multiple applications. Cadenaro et al. (2010) J. Am. Dent. Assoc. 2010;141:449–454.
- 101. • Efeoglu et al. (2005) used micro-computerized tomography to evaluate the effect of 10% carbamide peroxide applied to enamel. • Results indicated that the cause demineralization of the enamel extending to a depth of 50 μm below the enamel surface. • Therefore, they recommended that the application of bleaching agents should be carefully considered in patients susceptible to caries and tooth wear. Efeoglu N. et al. J. Dent. 2005;33:561–567
- 102. • Azrak et al. (2010) conducted an in vitro study to assess the effects of bleaching agents on eroded and sound enamel specimens and concluded that bleaching agents with a high concentration of peroxide or an acidic pH can provoke surface roughness of sound or eroded enamel. Azrak et al. J. Esthet. Restor. Dent. 2010;22:391–399.
- 103. Effects On Dentin: • Pecora et al. (1994) found that dentin microhardness decreased after the application of a 10% carbamide peroxide agent for 72 h. • Basting et al. (2003), results showed that the thickening agent (carbopol and/or glycerin), not just the 10% carbamide peroxide, caused a decrease in dentin microhardness. • Tam et al. (2005) concluded that direct exposure to 10% carbamide peroxide caused a significant decrease in the flexural strength and flexural modulus of bovine dentin. Tam L.E.et al J. Dent. 2005;33:451–458. Basting R.T.et al. J. Am. Dent. Assoc. 2003;134:1335–1342. Pecora J.D.,et al Braz. Dent. J. 1994;5:129– 134.
- 104. Effects On Dentin: • Lewinstein et al. (1994) showed a decrease in the microhardness of dentin following exposure to a 30% solution of hydrogen peroxide at pH 3. • Tam et al. (2007) found that in vitro fracture resistance of dentin was reduced after the prolonged use of bleaching products applied directly to dentin. Lewinstein I. et al J. Endod. 1994;20:61–63. Tam L.E. et al J. Esthet. Restor. Dent. 2007;19:100–109. discussion 110
- 105. Effects On Pulp: • Pulp penetration can occur within 5–15 minutes according to studies by Cooper et al. (1992). • Application of peroxide gel resulted in rapid penetration of peroxide to the pulp chamber. • Minor irritation of the pulp tissue may occur, but it resolves within 2 weeks after cessation of treatment.
- 106. • Camargo,SEA et al evaluated the pulp chamber penetration of peroxide bleaching agent in human and bovine teeth after office bleach technique. • Independent of the presence of restoration, all teeth submitted to bleaching presented peroxide penetration into the pulp chamber • The human teeth presented higher peroxide penetration than the bovine teeth • Peroxide penetration into the pulp chamber of restored teeth depends on the type of restorative material; it is higher in teeth restored with resin-modified glass ionomer cement. Camargo,SEA et al J Endod 2007;33:1074–1077)
- 107. • Patri G et al investigate the pulp chamber penetration of bleaching agent in intact teeth and teeth following restorative procedure. • They concluded that peroxide readily penetrates into the pulp through intact and restored teeth, with restored teeth showing higher pulpal peroxide levels than intact teeth. • Teeth restored with resin modified glass ionomer cement showed higher pulpal peroxide level than teeth restored with composite resins Patri G et al J Clin Diagn Res 2013 Dec; 7(12): 3057–3059
- 108. Tetracycline-stained Teeth When bleaching tetracycline-stained teeth, Haywood, Leonard and Dickinson,1997, were the first to show that using carbamide peroxide (CP) applied in trays and used overnight can be effective.
- 109. Two approaches have been used to treat tetracycline discoloration: (i) bleaching the external enamel surface (ii)Intracoronal bleaching following intentional root canal therapy. Kinoshita et al (2009) KTP laser, a type of Nd:YAG laser, for bleaching of tetracycline stained teeth. Effective Shorter time Simple chemical technique. No damage to the vital pulp and hard tissue crystals
- 110. Kwon et al (2012) Described the use of an extended whitening treatment of 2nd degree tetracycline stained teeth. Combination of in-office and home whitening provided a fast whitening result. Whitening of the upper arch for 8 weeks produced marked difference between the upper and lower teeth Post-treatment photograph, after a combination of in- office and home whitening for 16 weeks
- 111. Fluorosis and bleaching technique: Dental fluorosis, which is a hypomineralization of enamel due to the effects of excessive fluoride intake, results in white opaque areas or discolorations ranging from yellow to dark brown together with surface porosities on the enamel surface. Fluorosis staining is commonly considered an esthetic problem because of the psychological impact of unesthetic maxillary or mandibular anterior teeth
- 112. Guillen et al (2003) Used nightguard vital bleaching technique using carbamide and hydrogen peroxide as active agents Carbamide peroxide at 10 and 20% and hydrogen peroxide at 7.5% showed good clinical effectiveness in improving clinical appearence, but it is important to point out that clinical success is only in cases of class 1 to 3
- 113. Yildiz et al (2013) Described minimally invasive technique including enamel microabrasion followed by in-office bleaching The minimally invasive technique including enamel microabrasion and in-office bleaching was efficient After enamel microabrasion. Teeth after in- office bleaching. Severe Fluorosis tooth.
- 114. Effect of bleaching on restorative materials:
- 115. Restorative Material And Bleaching Agents: Amalgam Dental alloy Dental ceramic Glass ionomer cement Composite resin Effect of bleaching agents on bond strength of restorative materials to tooth structure .
- 116. Amalgam: Al-Salehi et al 2006 found no significant change in the release of metal ions from bleached amalgam (10% CP for 24 hours) Kasraei S et al 2010 reported a significant increase in the release of amalgam components (mercury and silver) after being exposed to CP (10-16%) and HP (3.6%, 6%, and 30%) for a longer treatment period.
- 117. Amalgam: El-Murr J et al 2011 reported that the concentration of mercury leaching from amalgam is below a level associated with possible health concerns. Schemehorn B et al reported that the greening of the tooth amalgam margin during extended 10% CP bleaching (7-10 months)
- 118. Dental alloy: Duschner H et al 2004 observed no significant deleterious effects of HP bleaching on gold alloy surfaces on surface microhardness . Tamam E et al in 2011 reported, Surface topographic alterations of gold, Ni-Cr, and Co-Cr alloys as a result of the application of 10% and 35% CP simulating at-home bleaching and in-office bleaching during 14 days, respectively.
- 119. Dental alloy: The HP bleaching agents (3%, 10%, and30%) caused increased corrosion potential of Ni-Cr and Pd-Cu-Ga alloys. Al-Salehi SK et al J Oral Rehabil 2008;35: 276e82.
- 120. Dental ceramic: Feldspathic porcelain exhibited surface deterioration in contact with 10% and 35% CP for 21 days. 35% CP induced a reduction in surface microhardness of both leucite reinforced and conventional glass ceramic. The increased roughness and whiteness of leached ceramic were possibly due to the reduction of surface SiO2 content. .. Moraes RR et al Clin Oral Investig 2006;10:23e8 Tu¨rker SB J Prosthet Dent 2003;89:466e73 Malkondu O¨Oper Dent 2011;36:177e86
- 121. Composite resin: 10% CP application for 3 weeks was able to change the surface roughness of packable composite resin. But the surface microhardness remained unchanged. Significant color changes of composite resin were observed, the possible explanation of bleaching-induced color changes of composite resin could be surface alteration and oxidation of the pigment. .. Basting R et al Esthet Restor Dent 2005;17: Torres CR et al Oper Dent 2012;37:526e31
- 122. • SEM studies and profilometric analyses, it was shown that 10–16% carbamide peroxide bleaching gels may lead to a slight, but statistically significant, increase in surface roughness and numbers of porosities of microfilled and hybrid composite resins Türker S.B.et al. J. Prosthet. Dent. 2003;89:466–473
- 123. • Hannig et al. (2007) reported a significant decrease in the surface hardness of bleached composite resins, not only on superficial surfaces, but also in the deeper layers of the filling materials. • These results were related to the high oxidation and degradation of the resinous matrix in the composites. Hannig C. et al Dent. Mater. 2007;23:198–203 Taher N.M.. J. Contemp. Dent. Pract. 2005;6:18–26
- 124. • Li et al. (2009) found significant changes in the color of nanohybrid and packable composite resins after bleaching with 15% carbamide peroxide. Li Q. et al J. Dent. 2009;37:348–356. • Generally, alterations in the color of restorative materials have been attributed to oxidation of surface pigments and amine compounds, which have also been indicated as responsible for color instability of restorative materials over time. Inokoshi S.et al. Oper. Dent. 1996;21:73–80.
- 125. • Differences in color change between and among different materials might be a result of different amounts of resin and different degrees of conversion of the resin matrix. • For the above-mentioned reasons, more color change was found in chemically cured than in light-cured composites, and this difference can be mainly attributed to the composition of the matrix phase, especially the activator system Inokoshi S.et al. Oper. Dent. 1996;21:73–80.
- 126. The effects of pre-operative dental bleaching on the bonding potential of composite resin restorations to tooth structure Dishman M.V et al reported decrease in bond strength to the presence of residual peroxide on the tooth surface, which interferes with the resin bonding and prevents its complete polymerization Perdigão J et al. reported that vital bleaching will alter the protein and mineral content of the superficial layers of enamel, which may be responsible for reduced bond strength Dishman M.V et al. Dent. Mater. 1994;10:33– 36 Perdigão J. et al. Am. J. Dent. 1998;11:291– 301.
- 127. • The SEM evaluation of bleached specimens, large areas of enamel surface were resin-free and tags were poorly defined and fragmented and penetrated to a lesser depth when compared with those in the unbleached control groups. • In another study, SEM examination of resin and bleached enamel interfaces displayed a porous and granular view with a ‘bubbly’ appearance Titley K.C. et al. J. Dent. Res. 1992;71:20–24. Titley K.C et al. J. Endod. 1991;17:72–75
- 128. • To improve the bond strength of previously bleached teeth, different methods have been proposed in the literature. • The effectiveness of 10% sodium ascorbate in reversing the compromised bond strength of enamel previously bleached with 10% carbamide peroxide when bonded to resin composite • Lai et al. (2002) found that surface treatment with an antioxidant (sodium ascorbate) can immediately reverse the compromised bond strength of teeth bleached with hydrogen peroxide or teeth treated with sodium hypochlorite Lai S.C et al. J. Dent. Res. 2002;81:477–481.
- 129. The effects of post-operative dental bleaching on the bonding potential of composite resin restorations to tooth structure The oxygen radicals released from peroxide bleaching materials are known for their high reactivity and nonspecific nature and may have side-effects on tooth tissues, restorative materials and the bond between them, which is usually the most susceptible to degradation. Barcellos et al. (2010) evaluated the effect of bleaching gel containing 10%, 15%, and 20% carbamide peroxide on the bond strength of dental enamel or dentin and resin composite restorations. Barcellos D.C.,et al Oper. Dent. 2010;35:463–469
- 130. • Results showed that carbamide peroxide bleaching agents could significantly affect the micro-tensile bond strength (μTBS) between the restoration and dental structure Barcellos D.C.,et al Oper. Dent. 2010;35:463–469 • Dudek et al. (2012) investigated the effect of peroxide bleaching gel on the durability of the adhesive bond among composite material, enamel, and dentin created with the etch-and-rinse adhesive Dudek M. et al. Oper. Dent. 2012;33:394–407.
- 131. • They concluded that the durability of adhesive restorations can be detrimentally influenced by carbamide peroxide bleaching, and that different adhesives show various levels of sensitivity to the bleaching gel. Dudek M. et al. Oper. Dent. 2012;33:394–407.
- 132. Conclusion: The increasing demand for tooth bleaching has driven many manufacturers and researchers to develop bleaching products to be used either in the dental office or at home. However, as with any dental procedure, bleaching involves risks. To minimize the risks, the involvement of dental professionals, the prevention of using of OTC bleaching products and the reduction of overused of bleaching products are necessary.
- 133. Conclusion: The dentist must understand the differences amongst the currently available bleaching techniques and the difference in concentration of various products.
- 134. Thank you
- 135. References:
- 136. • INGLE’s Endodontics - 6th edition. • GROSSMAN’s Endodontic practice - 12th edition • Linda Greenwall. CRC Press; 2017 Tooth whitening techniques, second edition. • Nutter BJ, Sharif MO, Smith AB, Brunton PA.A clinical study comparing the efficacy of light activated in-surgery whitening versus in-surgery whitening without light activation. J Dent. 2013 Nov; 41 Suppl 5():e3-7. • Hahn P, Schondelmaier N, Wolkewitz M, Altenburger MJ, Polydorou O. Efficacy of tooth bleaching with and without light activation and its effect on the pulp temperature: an in vitro study. Odontology. 2013 Jan; 101(1):67-74. • GR, Borges AB, Cassiano KV, Pucci CR. Assessment of the effectiveness of light-emitting diode and diode laser hybrid light sources to intensify dental bleaching treatment.Torres CR, Barcellos DC, Batista Acta Odontol Scand. 2011 May; 69(3):176-81.
- 137. • Azrak B., Callaway A., Kurth P., Willershausen B. Influence of bleaching agents on surface roughness of sound or eroded dental enamel specimens. J. Esthet. Restor. Dent. 2010;22:391–399. • Smidt A., Feuerstein O., Topel M. Mechanical, morphologic, and chemical effects of carbamide peroxide bleaching agents on human enamel in situ. Quintessence Int. 2011;42:407–412 • Cadenaro M., Navarra C.O., Mazzoni A. An in vivo study of the effect of a 38 percent hydrogen peroxide in-office whitening agent on enamel. J. Am. Dent. Assoc. 2010;141:449–454. • He LB, Shao MY, Tan K, Xu X, Li JY. The effects of light on bleaching and tooth sensitivity during in-office vital bleaching: a systematic review and meta-analysis. J Dent. 2012 Aug; 40(8):644-53.
- 138. • Domínguez A, García JA, Costela A, Gómez C. Influence of the light source and bleaching gel on the efficacy of the tooth whitening process. Photomed Laser Surg. 2011 Jan; 29(1):53-9. • Hein DK, Ploeger BJ, Hartup JK, Wagstaff RS, Palmer TM, Hansen LD. In-office vital tooth bleaching--what do lights add? Compend Contin Educ Dent. 2003 Apr; 24(4A):340-52. • Kwon R.Review of the Mechanism of Tooth Whitening.J Esthet Restor Dent 2015;27:240–257. • Pecora J.D., Cruzfilho A.M., Sousaneto M.D., Silva R.G. In vitro action of various bleaching agents on the microhardness of human dentin. Braz. Dent. J. 1994;5:129–134.
- 139. • Pecora J.D., Cruzfilho A.M., Sousaneto M.D., Silva R.G. In vitro action of various bleaching agents on the microhardness of human dentin. Braz. Dent. J. 1994;5:129–134. • Tam L.E., Lim M., Khanna S. Effect of direct peroxide bleach application to bovine dentin on flexural strength and modulus in vitro. J. Dent. 2005;33:451–458. • Basting R.T., Rodrigues A.L., Jr., Serra M.C. The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time. J. Am. Dent. Assoc. 2003;134:1335–1342. • Tam L.E., Kuo V.Y., Noroozi A. Effect of prolonged direct and indirect peroxide bleaching on fracture toughness of human dentin. J. Esthet. Restor. Dent. 2007;19:100–109. discussion 110.
Editor's Notes
- Classification Based on Chemistry of Staining put forth by Nathoo [1997] : a) N1 Type or Direct Dental Stain: The coloured materials (chromogens) bind to the tooth surface & cause discolouration. The colour of the dental stain is same as the colour of the chromogens. b) N2 Type or Direct Dental Stain: The chromogens change colour after binding to the tooth. This is actually N1 type of food stain darkens with time. c) N3 type or Indirect Dental Stain: Colourless materials or a prechromogen binds to the tooth & undergoes chemical reaction to cause a stain
- Bleaching defination types of bleching
- Chlorine acts indirectly as it is capable of releasing the oxygen from a water molecule. Cl2 + H2o = 2HCl + 1/2O2 enamel and dentin are expected to act as semipermeable membranes and that they allow hydrogen peroxide to move according to Fick’s second law of diffusion, which describes that the diffusion of a molecule is proportional to the surface area, diffusion coefficient, and concentration, and that it is inversely proportional to the diffusion distance
- Trolamine, which is a neutralizing agent, is often added to Carbopol to reduce the pH of the gels to 5–7.
- Reducing agent Oxidising agent Tooth Bleaching material After the process Tooth is oxidized Bleaching material is reduced (Organic pigmentation of tooth oxidized)
- Peroxide solutions flow freely through the enamel and dentin owing to the porosity and permeability of these structures. The free movement is caused by the relatively low molecular weight of the peroxide molecule and the penetrating nature of the oxygen and superoxide radicals.
- Old McInnes Ratio New McInnes Ratio Bleaching enamel a) 30% H2 O2 5 parts 30% H2 O2 1 part Etches enamel b) 36% HCl 5 parts Removes surface debris 0.2% ether 1 part 20% NaOH 1 part
- If patients do not wear the whitening agent in the trays for the specified amount of time, changes in tooth lightening will be slow. • Some patients cannot be bothered with applying the whitening agent in the trays every day. The dropout rate for home whitening may be as much as 50% according to anecdotal feedback.
- contraindicationS to uSe There are many contraindications to home whitening (Greenwall 1999b). Home whitening agents should not be used in the following situations: • Severe tetracycline staining. • Severe pitting hypoplasia. • Severe fluorosis stain. • Discolorations in the adolescent patient with large pulps (Haywood 1995). • Patients with unrealistic expectations about the anticipated esthetic result (Wise 1995). • Teeth with inadequate or defective existing restorations (these should be temporarily blocked before whitening). • Teeth with tooth surface loss from attrition, abrasion, and severe erosion. • Teeth with insufficient enamel to respond to whitening (i.e., pitted teeth, defective enamel); however, this might be acceptable because it is the dentin that is important for determining the shade color (Bentley et al. 1999). • Teeth with deep and surface cracks and fracture lines (see Figure 5.22A). • Teeth with large anterior restorations that have existing sensitivity. • Teeth with pathology such as a periapical radiolucency. • Teeth that are fractured or misaligned may be better treated with other treatments such as porcelain veneers or orthodontics. Further treatment may be necessary. • Patients who demonstrate a lack of compliance through inability or unwillingness to wear appliance for the required time (Garber et al. 1991). • Patients who are pregnant or lactating—at this stage, the effect of the whitening agent on development of the fetus is unknown (Garber et al. 1991). • Patients who smoke—patients cannot smoke and whiten their teeth at the same time because this may enhance the carcinogenic effect of the smoking (see Figure 5.20). • Teeth exhibiting extreme sensitivity to heat, cold, touch, and sweetness.
- contraindicationS to uSe There are many contraindications to home whitening (Greenwall 1999b). Home whitening agents should not be used in the following situations: • Severe tetracycline staining. • Severe pitting hypoplasia. • Severe fluorosis stain. • Discolorations in the adolescent patient with large pulps (Haywood 1995). • Patients with unrealistic expectations about the anticipated esthetic result (Wise 1995). • Teeth with inadequate or defective existing restorations (these should be temporarily blocked before whitening). • Teeth with tooth surface loss from attrition, abrasion, and severe erosion. • Teeth with insufficient enamel to respond to whitening (i.e., pitted teeth, defective enamel); however, this might be acceptable because it is the dentin that is important for determining the shade color (Bentley et al. 1999). • Teeth with deep and surface cracks and fracture lines (see Figure 5.22A). • Teeth with large anterior restorations that have existing sensitivity. • Teeth with pathology such as a periapical radiolucency. • Teeth that are fractured or misaligned may be better treated with other treatments such as porcelain veneers or orthodontics. Further treatment may be necessary. • Patients who demonstrate a lack of compliance through inability or unwillingness to wear appliance for the required time (Garber et al. 1991). • Patients who are pregnant or lactating—at this stage, the effect of the whitening agent on development of the fetus is unknown (Garber et al. 1991). • Patients who smoke—patients cannot smoke and whiten their teeth at the same time because this may enhance the carcinogenic effect of the smoking (see Figure 5.20). • Teeth exhibiting extreme sensitivity to heat, cold, touch, and sweetness.
- The vitality of the teeth should be tested, particularly single discolored teeth. Is nonvital rct
- This is normally two shades lighter on a normal porcelain shade guide (Vita Classic shade guide) or 1–3 shades lighter on a value-oriented shade guide (Vita 3D master).
- This is normally two shades lighter on a normal porcelain shade guide (Vita Classic shade guide) or 1–3 shades lighter on a value-oriented shade guide (Vita 3D master).
- This is normally two shades lighter on a normal porcelain shade guide (Vita Classic shade guide) or 1–3 shades lighter on a value-oriented shade guide (Vita 3D master).
- The ideal whitening trays should: • Be strong enough to avoid damage by the patient during wear. • Not distort during use. • Not wear during use. • Be easy to fit and easy to remove after a treatment session. • Be made from a material that is bioinert (Greenwall 1999). • Not cause irritation to the soft tissues, gingivae,mucosa, tongue, or teeth. • Not impinge too far on the papillae. • Be thin enough to be well tolerated in the mouth. • Be smooth and well polished so that there are no rough edges. • Fit comfortably and passively and not feel too tight in places. • Not extend into deep undercuts. • Be correctly trimmed with freedom of movement for the frenum attachments if the full vestibule design is used. • Have good retention. • Be easy to clean and rinse. • Not distort during storage.
- The ideal whitening trays should: • Be strong enough to avoid damage by the patient during wear. • Not distort during use. • Not wear during use. • Be easy to fit and easy to remove after a treatment session. • Be made from a material that is bioinert (Greenwall 1999). • Not cause irritation to the soft tissues, gingivae,mucosa, tongue, or teeth. • Not impinge too far on the papillae. • Be thin enough to be well tolerated in the mouth. • Be smooth and well polished so that there are no rough edges. • Fit comfortably and passively and not feel too tight in places. • Not extend into deep undercuts. • Be correctly trimmed with freedom of movement for the frenum attachments if the full vestibule design is used. • Have good retention. • Be easy to clean and rinse. • Not distort during storage.
- The fluoride works by blocking the tubules. The potassium nitrate reduces sensitivity via chemical interference that prevents the pulpal sensory nerve from repolarizing after initial depolarization.
- If patients do not wear the whitening agent in the trays for the specified amount of time, changes in tooth lightening will be slow. • Some patients cannot be bothered with applying the whitening agent in the trays every day. The dropout rate for home whitening may be as much as 50% according to anecdotal feedback.
- Old McInnes Ratio New McInnes Ratio Bleaching enamel a) 30% H2 O2 5 parts 30% H2 O2 1 part Etches enamel b) 36% HCl 5 parts Removes surface debris 0.2% ether 1 part 20% NaOH 1 part
- Lights may accelerate the release of hydroxyl-radicals from peroxide in two ways: one is photolysis, the other is thermocatalysis. commercial bleaching lamps emit light falling within the visible spectrum, and their use may involve little photolysis. light is projected onto the bleaching agents, a fraction of light may be mainly transmitted as heat to degrade the peroxide after being absorbed by agents.12,13 Thus, the advantage of using light in tooth bleaching is to ‘heat’ HP. In other words, thermocatalysis may be the main mechanism of light activation.
- Beyond Polus Whitening Accelerator Accelerator is a multi-functional, halogen-powered whitening lamp with LightBridge technology (combines halogen and LED light technologies), LED curing light and spot whitening device, and optional low-level laser therapy treatment device.
- The Zoom!™ Chairside Teeth Whitening System (Discus Dental, Inc., Culver City, CA, USA) is one power bleaching system that consists of a mercury halide lamp filtered to emit light in the 350-400 nm range
- The pH of the whitening agent, The method of application and thickness of the whitening agent applied to the enamel The fluctuation of light irradiation The length of photoactivation Tooth size Selective absorption of the wavelength of light.
- Vitamin E oil (α-tocopherol), a fat-soluble antioxidant, may neutralize accidental soft tissue contact with the peroxide.
- If patients do not wear the whitening agent in the trays for the specified amount of time, changes in tooth lightening will be slow. • Some patients cannot be bothered with applying the whitening agent in the trays every day. The dropout rate for home whitening may be as much as 50% according to anecdotal feedback.
- contraindicationS to uSe There are many contraindications to home whitening (Greenwall 1999b). Home whitening agents should not be used in the following situations: • Severe tetracycline staining. • Severe pitting hypoplasia. • Severe fluorosis stain. • Discolorations in the adolescent patient with large pulps (Haywood 1995). • Patients with unrealistic expectations about the anticipated esthetic result (Wise 1995). • Teeth with inadequate or defective existing restorations (these should be temporarily blocked before whitening). • Teeth with tooth surface loss from attrition, abrasion, and severe erosion. • Teeth with insufficient enamel to respond to whitening (i.e., pitted teeth, defective enamel); however, this might be acceptable because it is the dentin that is important for determining the shade color (Bentley et al. 1999). • Teeth with deep and surface cracks and fracture lines (see Figure 5.22A). • Teeth with large anterior restorations that have existing sensitivity. • Teeth with pathology such as a periapical radiolucency. • Teeth that are fractured or misaligned may be better treated with other treatments such as porcelain veneers or orthodontics. Further treatment may be necessary. • Patients who demonstrate a lack of compliance through inability or unwillingness to wear appliance for the required time (Garber et al. 1991). • Patients who are pregnant or lactating—at this stage, the effect of the whitening agent on development of the fetus is unknown (Garber et al. 1991). • Patients who smoke—patients cannot smoke and whiten their teeth at the same time because this may enhance the carcinogenic effect of the smoking (see Figure 5.20). • Teeth exhibiting extreme sensitivity to heat, cold, touch, and sweetness.
- In an in vitro study (McCaslin et al. 1999) using 10% carbamide peroxide placed directly onto the enamel to validate the color change in dentin and to assess whether dentin changed uniformly, it was noted that a color change occurred throughout the dentin and the color change was uniform
- In an in vitro study (McCaslin et al. 1999) using 10% carbamide peroxide placed directly onto the enamel to validate the color change in dentin and to assess whether dentin changed uniformly, it was noted that a color change occurred throughout the dentin and the color change was uniform
- Possible side effects of whitening agents Gingivae • Tissue sloughing • Gingival irritation • Gingival ulceration • Change in gingival texture • Gingival soreness • Whitening of the maxillary papillae • Possible gingival irritation if the tray is overextended • Possible opening of the black triangles and enlargement of black spaces Teeth External effects • Uneven, incomplete whitening, streaky appearance • White spots or banding within the tooth may be more noticeable • A demarcation line may be visible between the color on the incisal tip and the cervical neck • Snow-capped appearance on the lower incisors as a result of slower whitening Internal effects Pulp • Transient thermal sensitivity • Flare-up of an existing quiescent apical area Oral mucosa • Sore throat • Unpleasant taste • Burning palate • Pain and sensitivity • Ulceration • Soft tissue lacerations Other • Irritation of the tongue from rough edges of the whitening tray • Mild laxative effect • Gastric irritation • Allergy, facial swelling or petechiae on face and neck
- well-documented side-effects of tetracycline use is it‟s incorporation as a fluorescent pigment into tissues that are calcifying at the time of administration. • It has the ability to chelate calcium ions and to be incorporated into teeth, cartilage and bone, to form a tetracycline-calcium orthophosphate complex (Eisenberg 1975) resulting in discoloration and enamel hypoplasia of both the primary and permanent dentitions if administered during the period of tooth development. • The severity of the discolouration is considered to be related to dose, frequency, duration of therapy and critically the stage of odontogenesis. First degree staining: minimal, uniformly distributed, light yellow, light brown or light grey discoloration, restricted to three quarters of the incisal part of the crown. Good treatment prognosis. Second degree staining: staining varies more in quantity and location, ranging from deep yellow to brown or grey with no banding. Treatment prognosis is variable, as it depends entirely on the intensity of the staining. Third degree staining: dark brown, dark grey, purple or blue staining with marked banding. Prognosis for an efficient and aesthetic outcome is not good, although teeth may be lightened to some degree. Fourth degree staining: intense pigmentation combining very dark stains with highly pronounced bands. Bleaching is inefficient in such cases
- Karium-Titanium-Phosphoric acid
- Karium-Titanium-Phosphoric acid
- a minimally invasive technique (enamel microabrasion and in-office bleaching) was used for the management of severely fluorosed teeth. Enamel microabrasion improved the appearance of teeth by removing brown stains and enamel porosities while in-office bleaching provided further esthetic improvement by removing residual brown stains and producing a whiter and more homogenous tooth structure. A slight staining was observed at the 2-year follow-up, but the clinical appearance of teeth was acceptable and patient satisfaction was considerably high