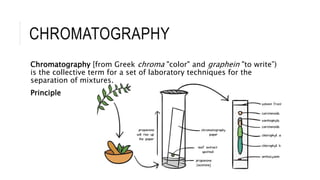
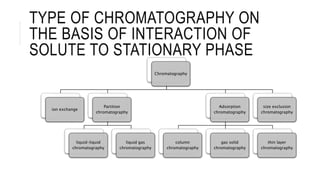
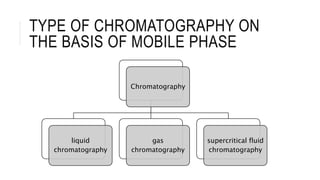
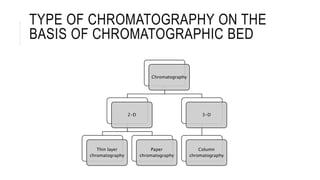
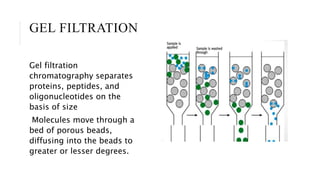
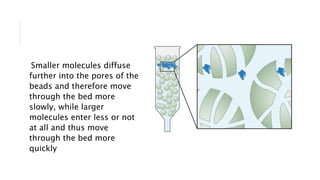
Chromatography is a laboratory technique for separating mixtures based on the different distribution coefficients of their components. Gel filtration chromatography specifically separates molecules like proteins and peptides based on size, utilizing a bed of porous beads where smaller molecules move slower due to diffusion into the pores. Factors affecting resolution include column length, diameter, flow rate, and properties of the resin used.