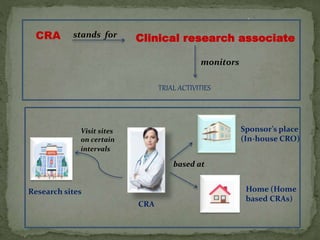
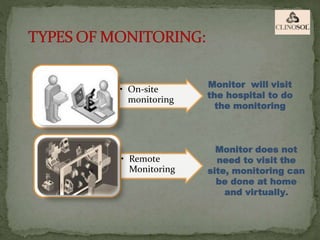
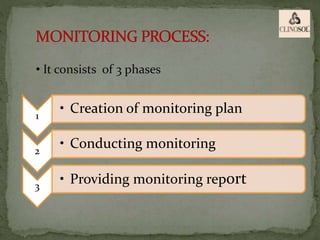
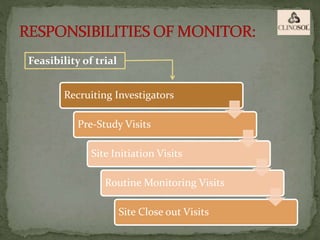
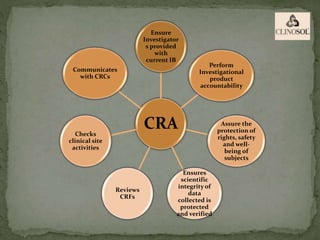
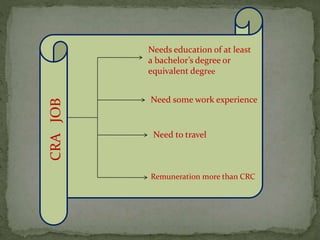
The document outlines the roles and responsibilities of Clinical Research Associates (CRAs) in monitoring clinical trials, including types of monitoring such as on-site and remote. It details the monitoring process, from pre-study visits to site closure and emphasizes the importance of ensuring subject safety and data integrity. CRAs require at least a bachelor's degree, travel for work, and often receive higher remuneration than Clinical Research Coordinators (CRCs).