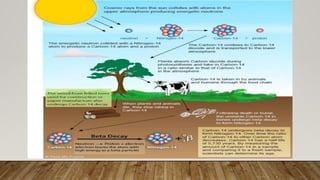
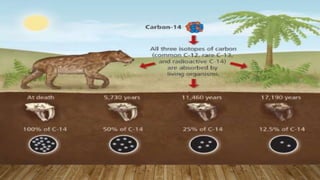
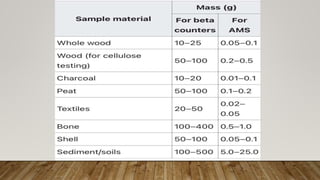
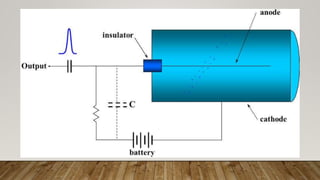
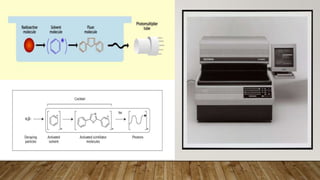
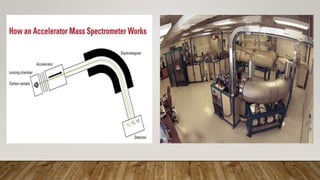
Radiocarbon dating is a method for determining the age of organic material by measuring the amount of radioactive carbon-14 remaining in the sample. Willard Libby developed the method in the 1940s, for which he won the Nobel Prize in 1960. It is based on the principle that living organisms absorb carbon-14 from the atmosphere, but after death, the amount of carbon-14 decreases as it radioactively decays. By measuring the amount of carbon-14 remaining and knowing its half-life of 5730 years, the age of the sample can be estimated. Radiocarbon dating has revolutionized understanding of chronologies in archaeology, geology, and other fields, but is limited to dating samples under 60,000 years