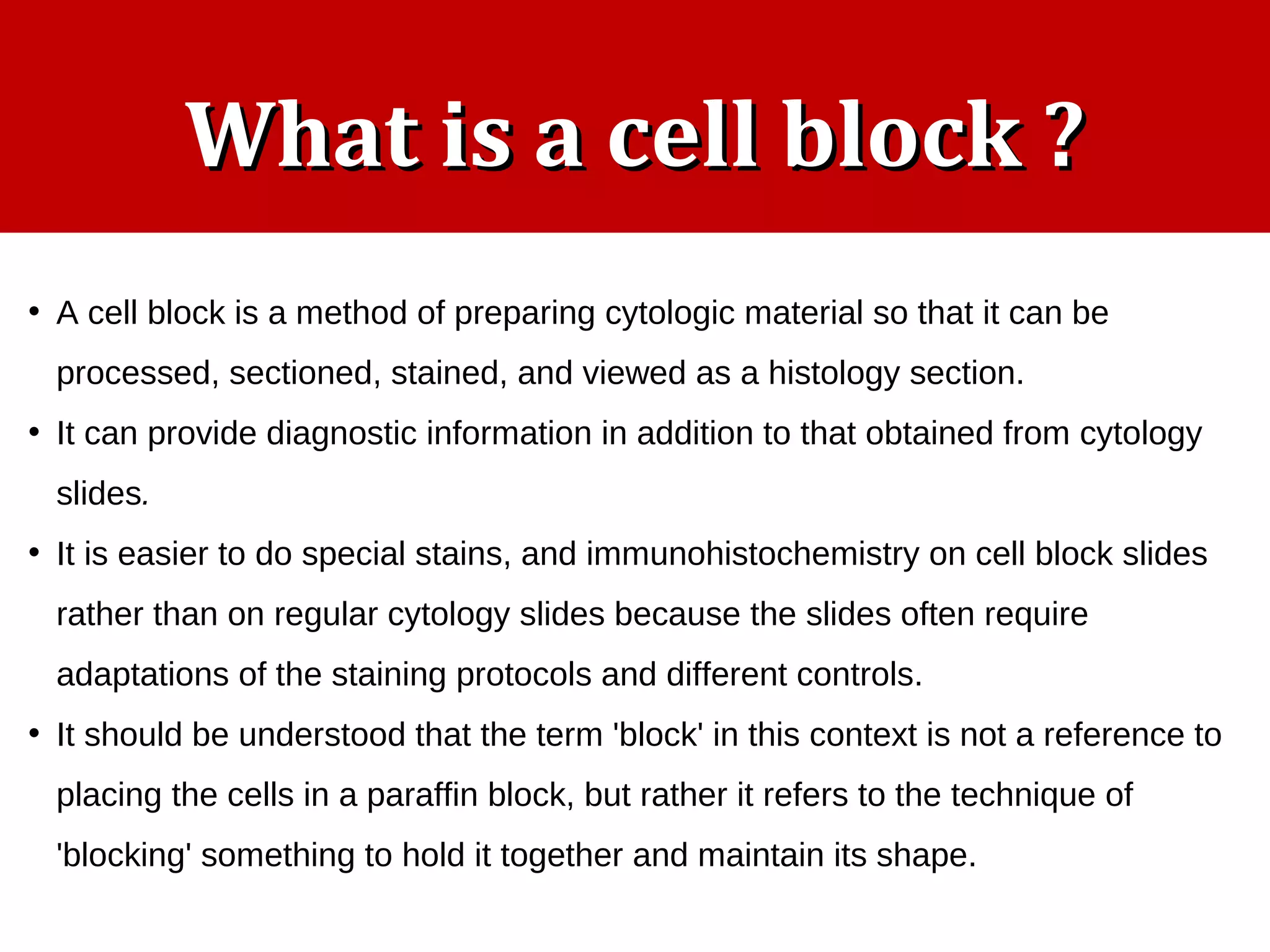
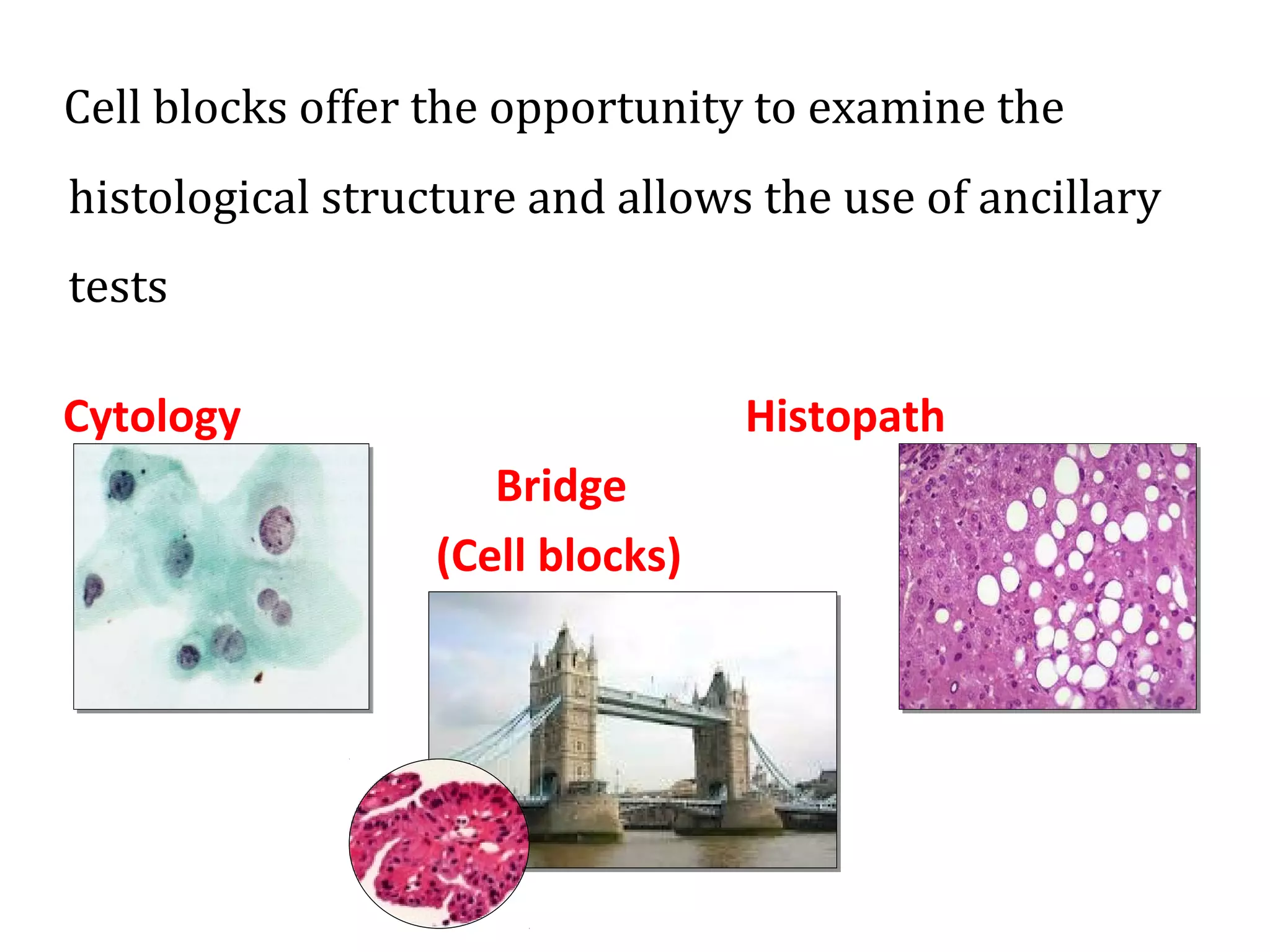
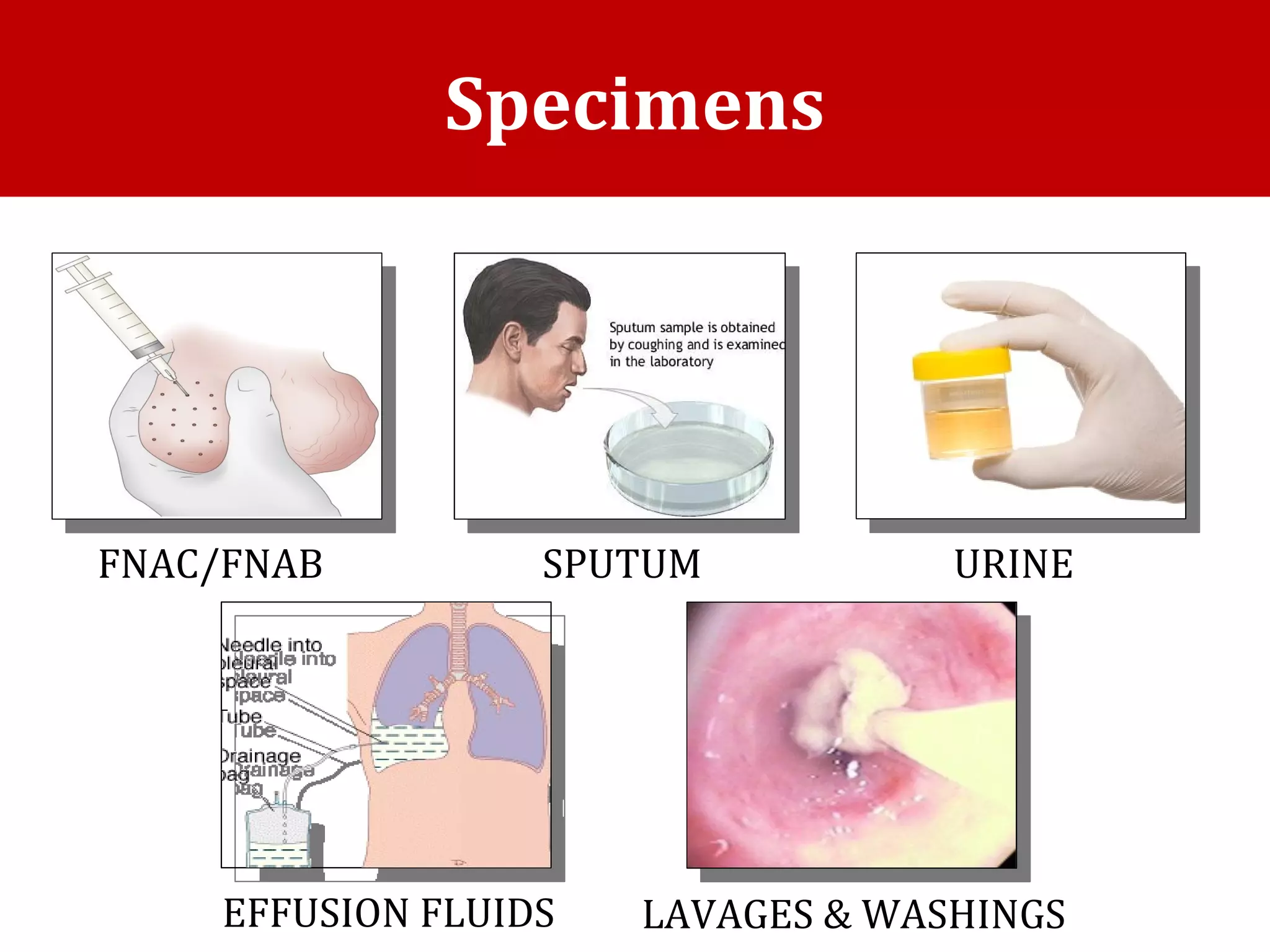
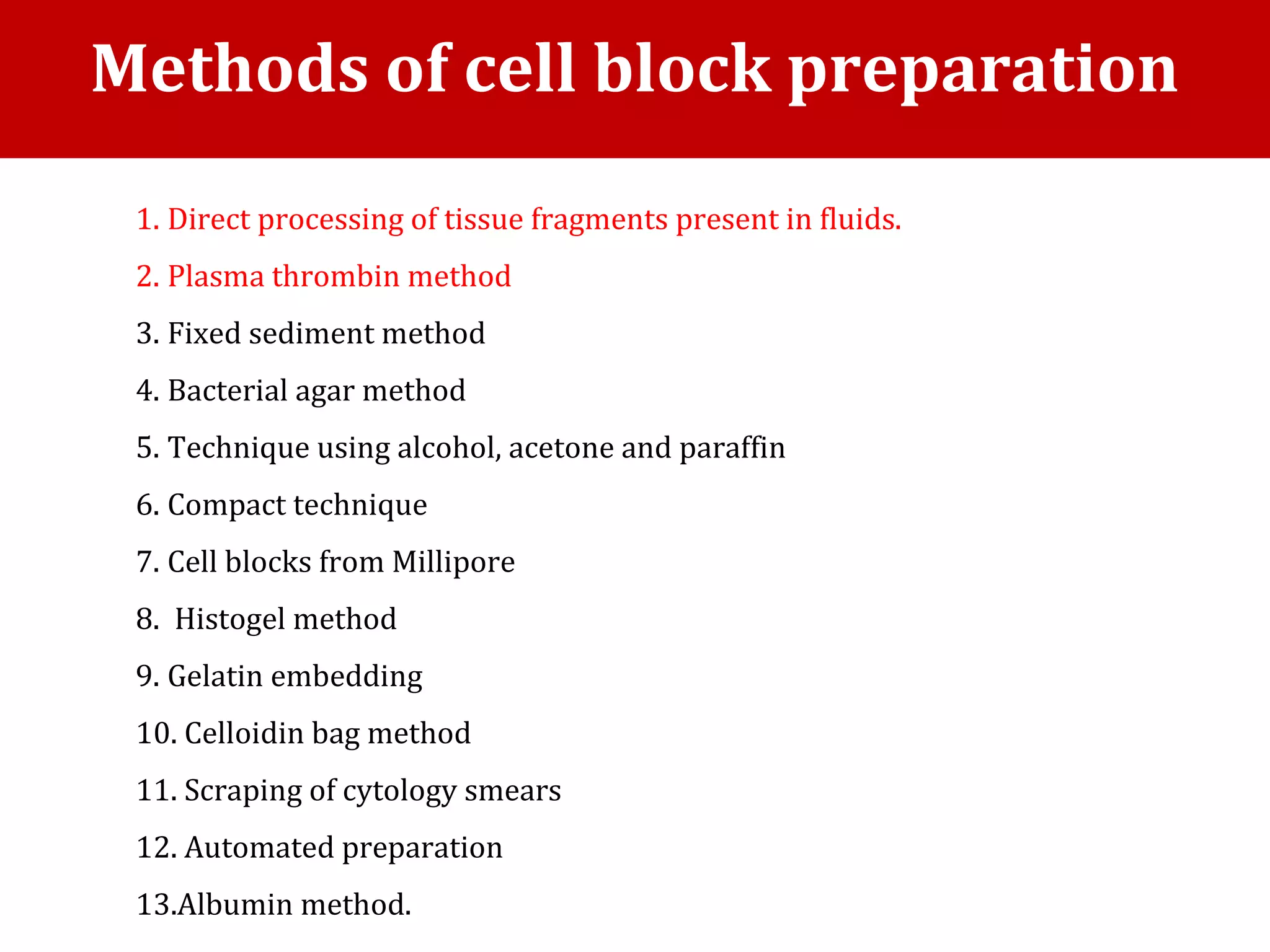
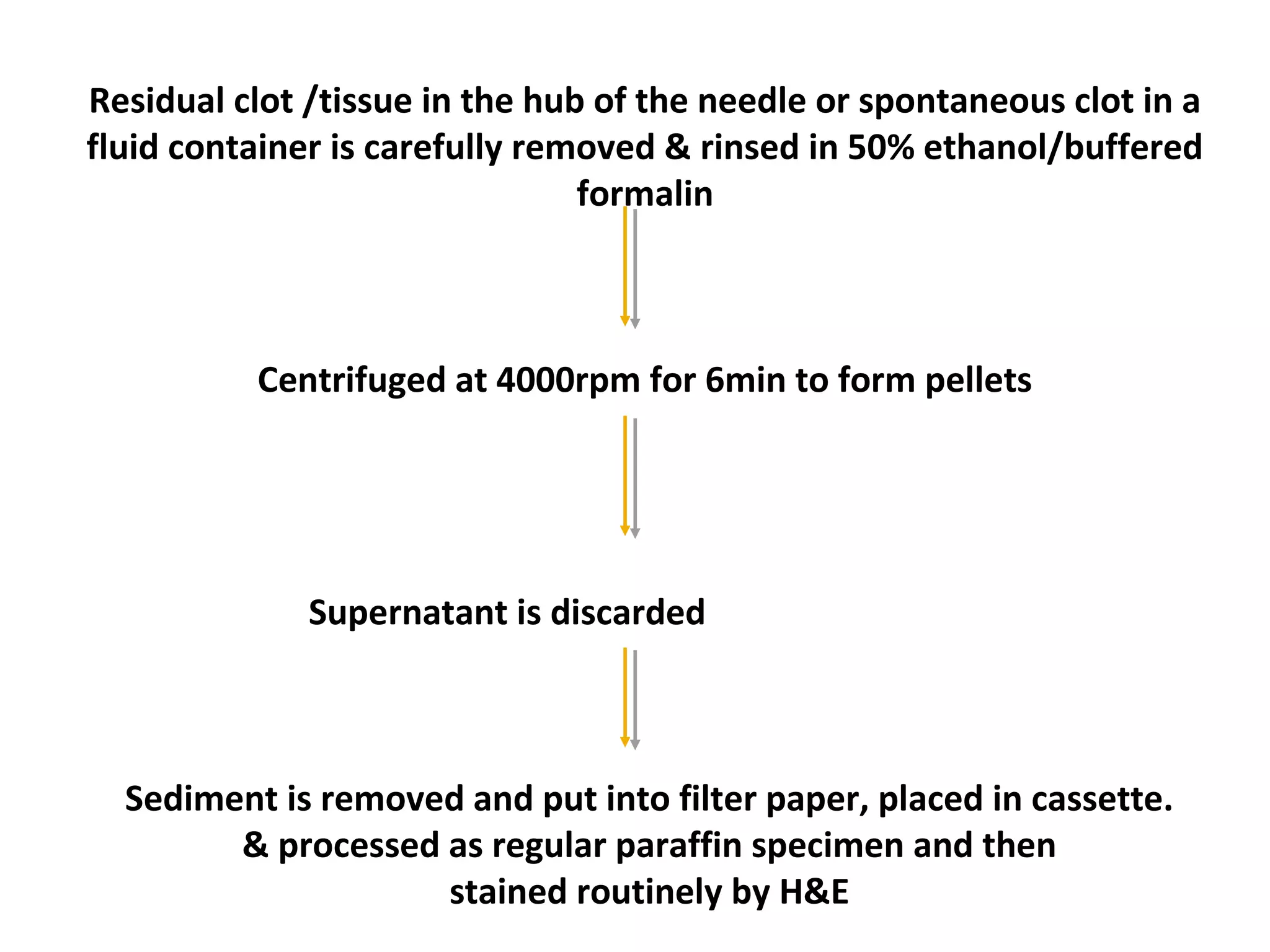
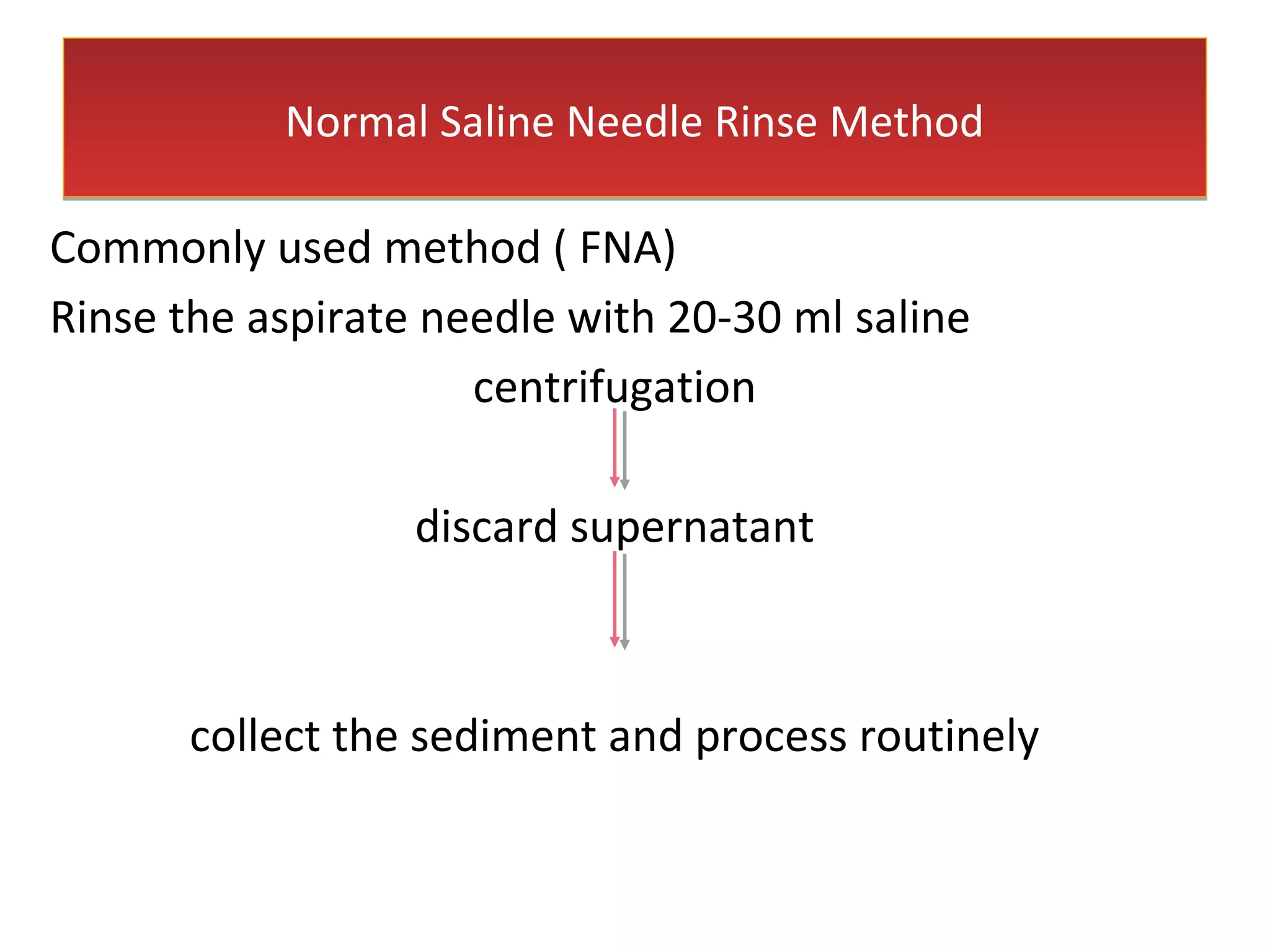
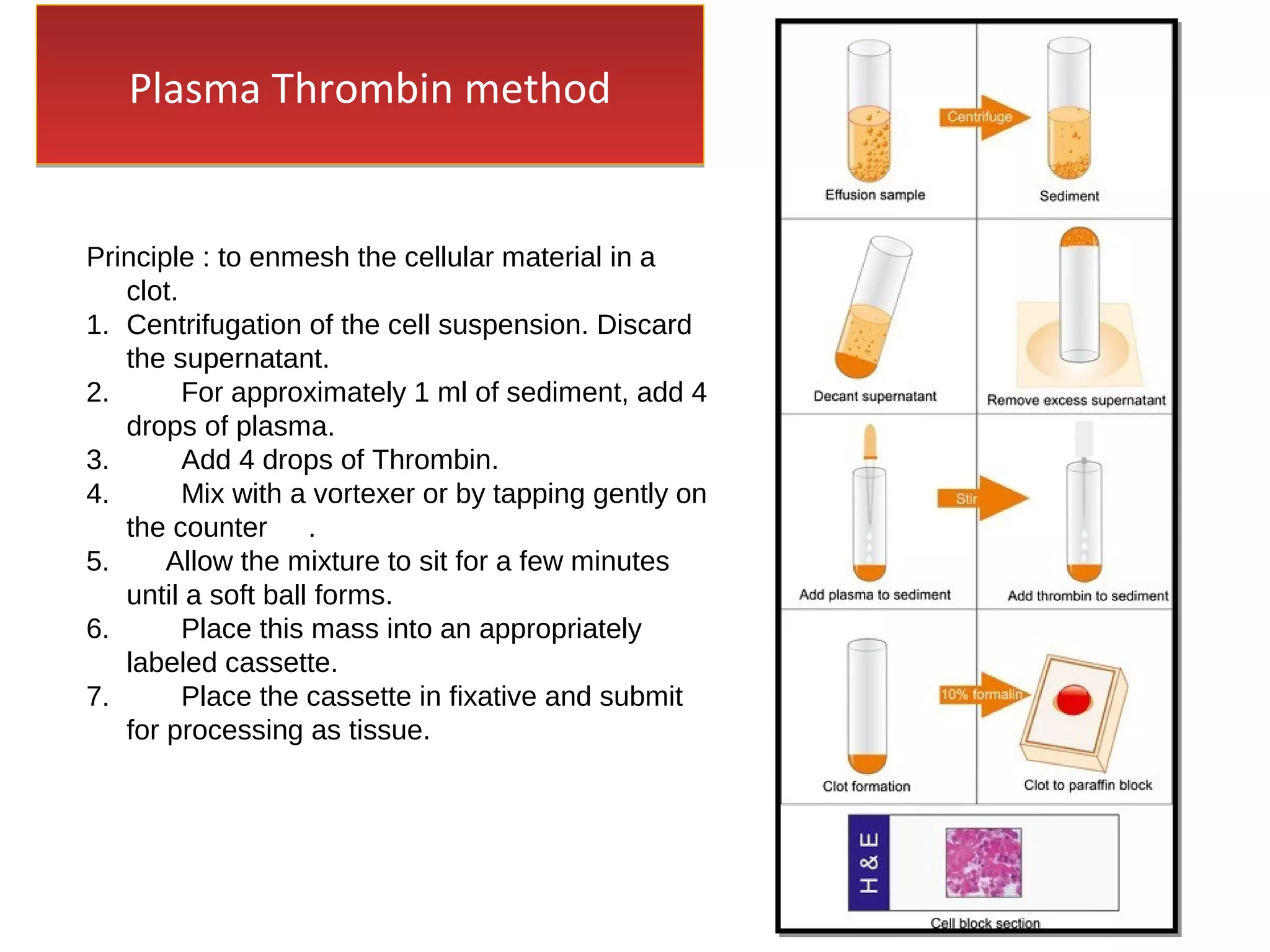
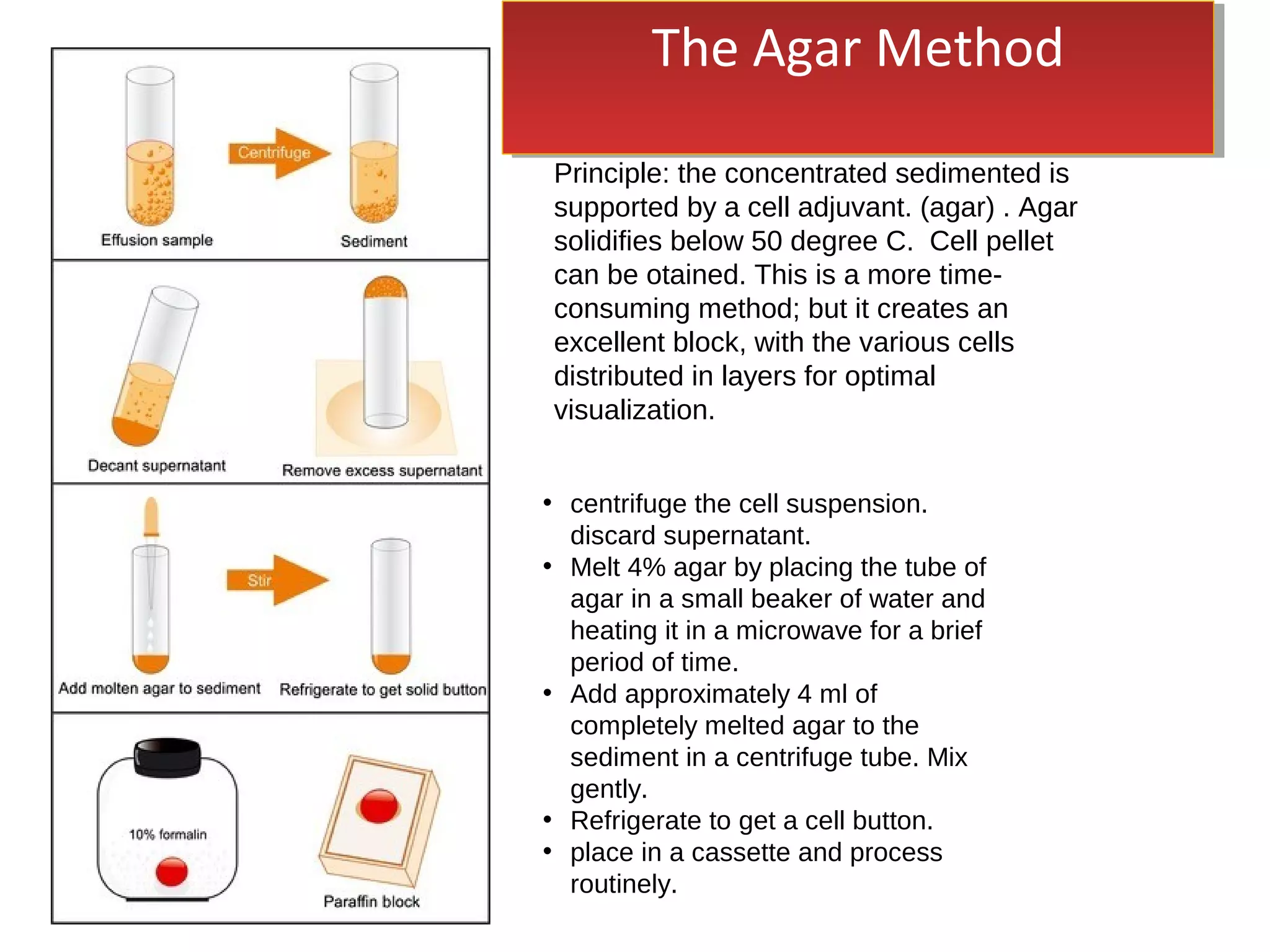
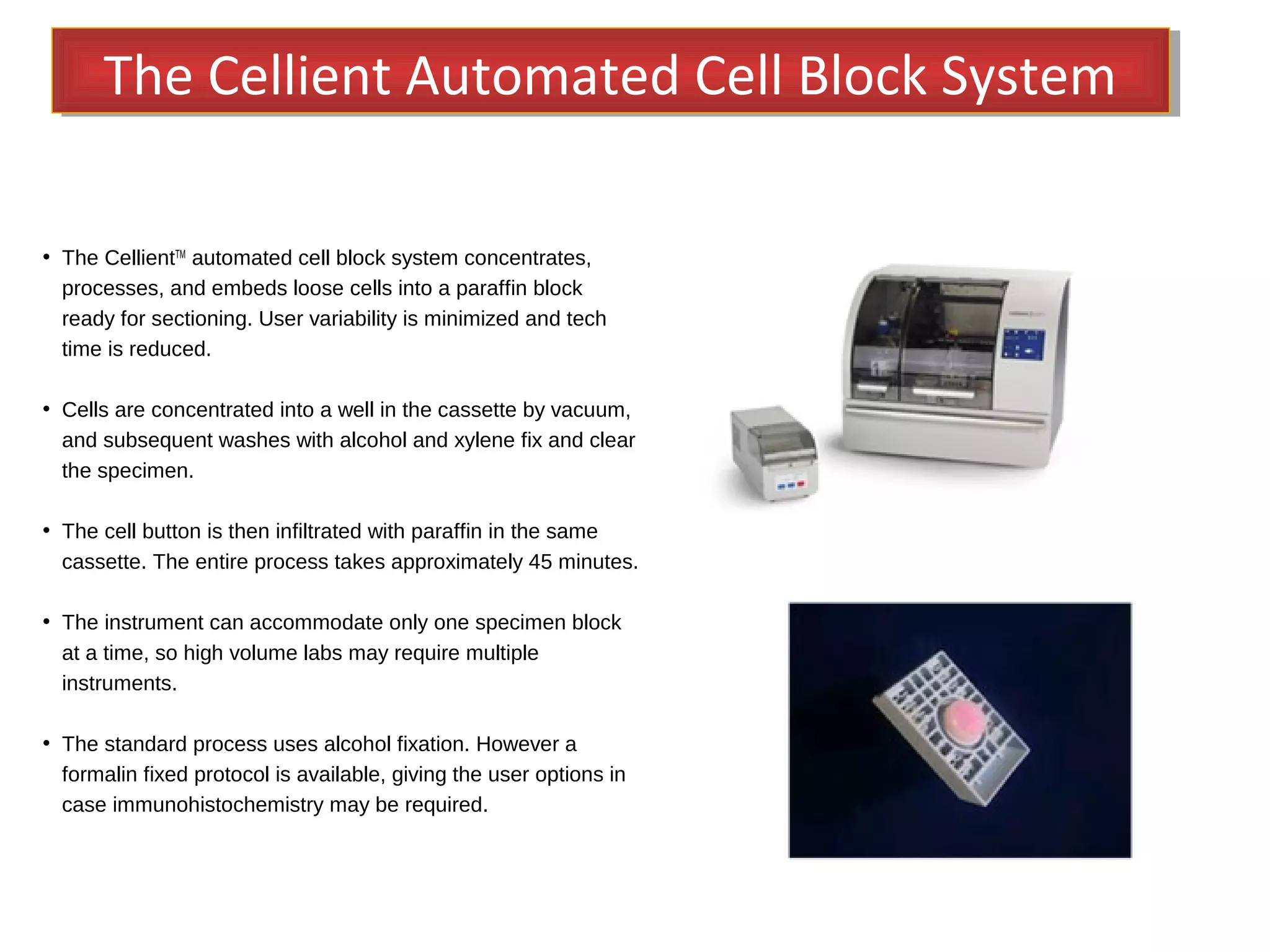
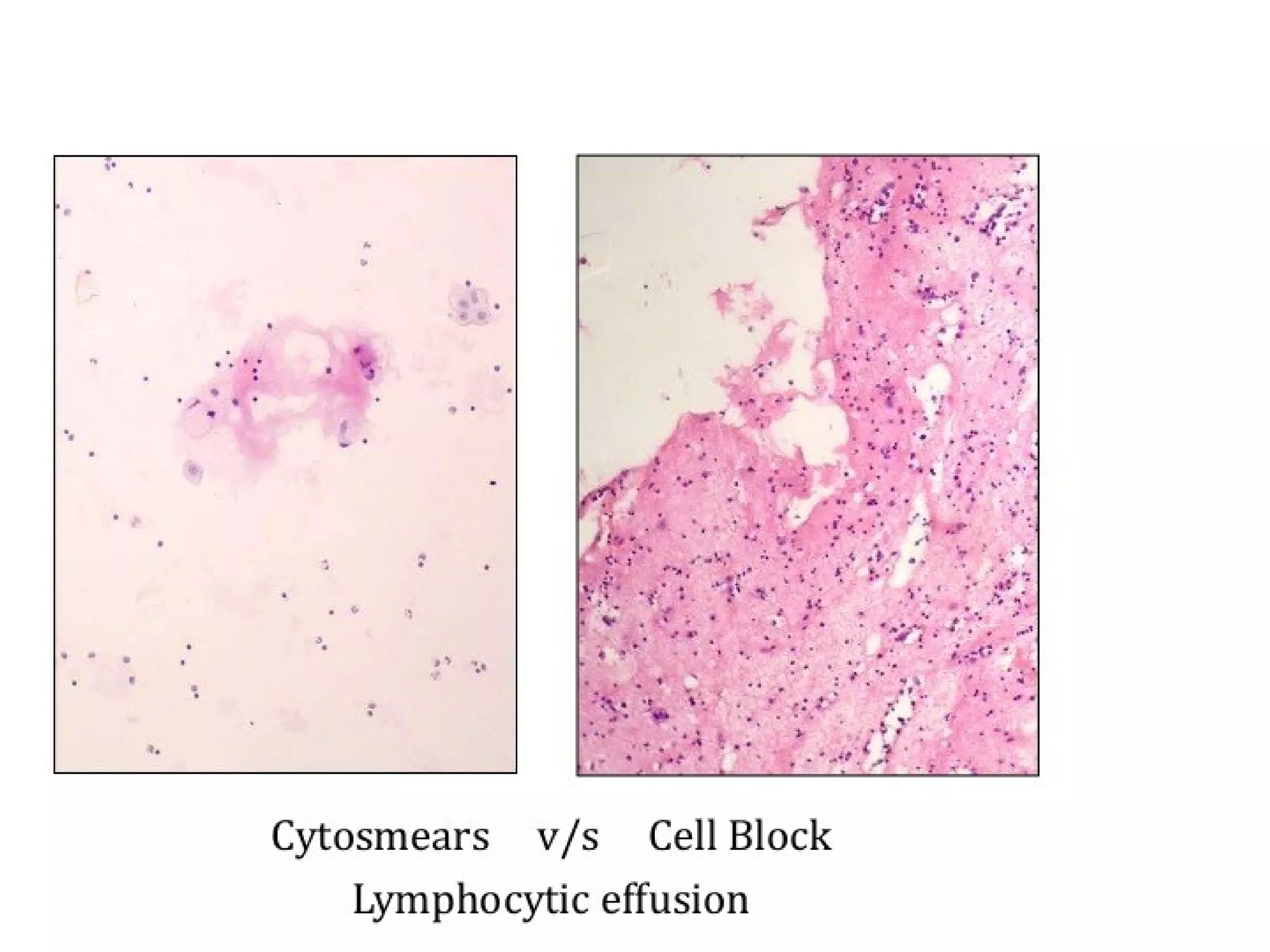
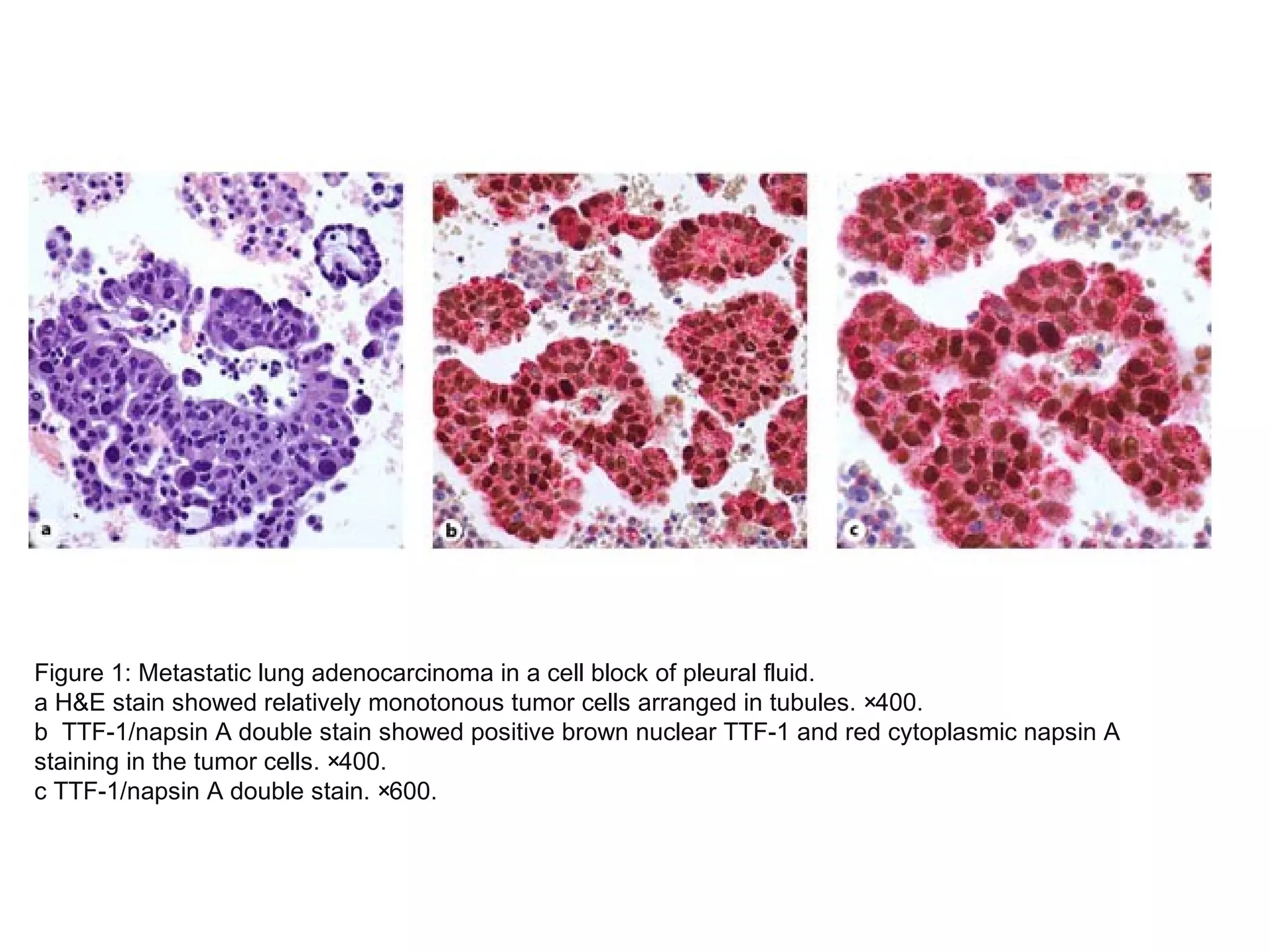
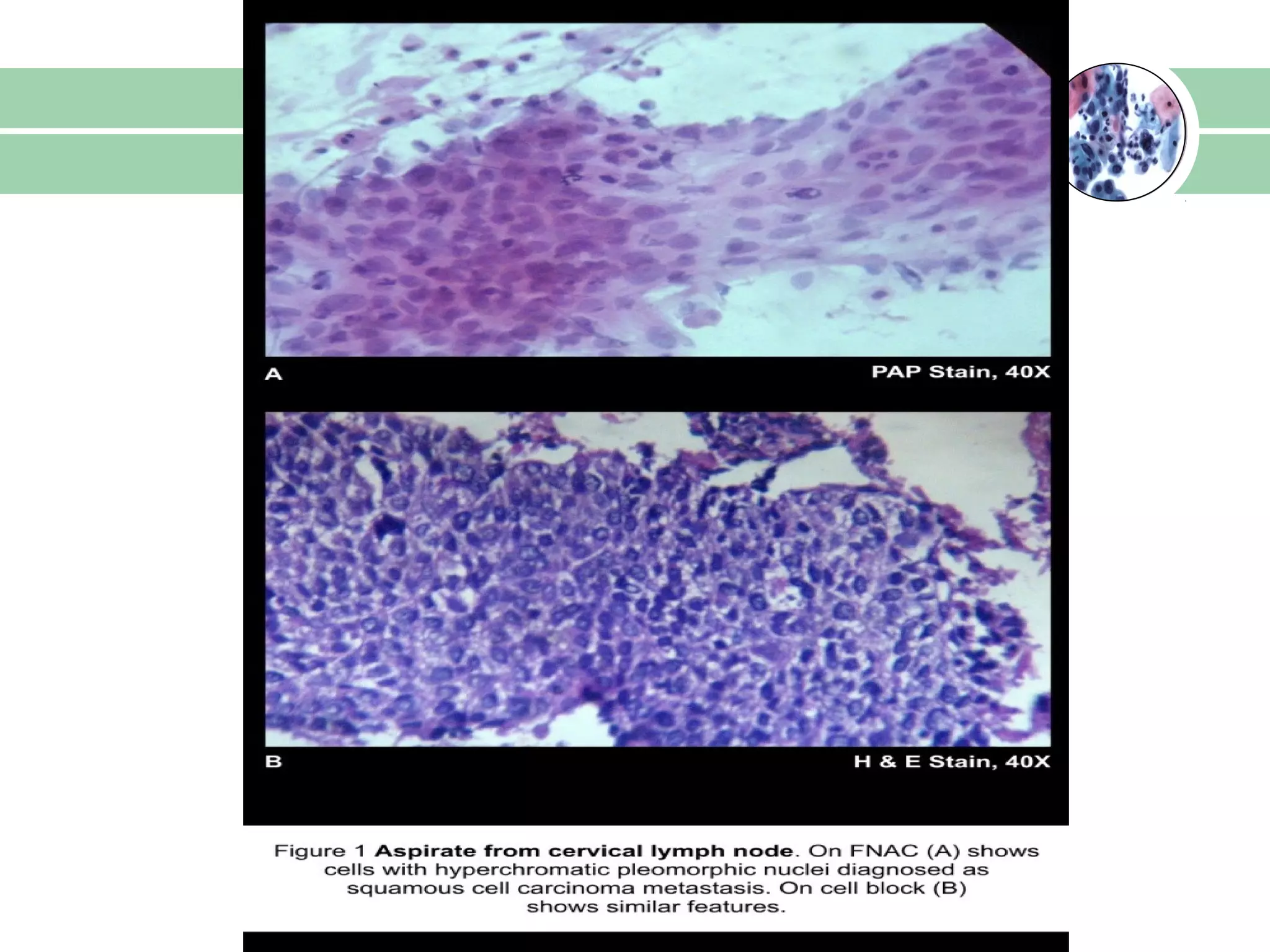
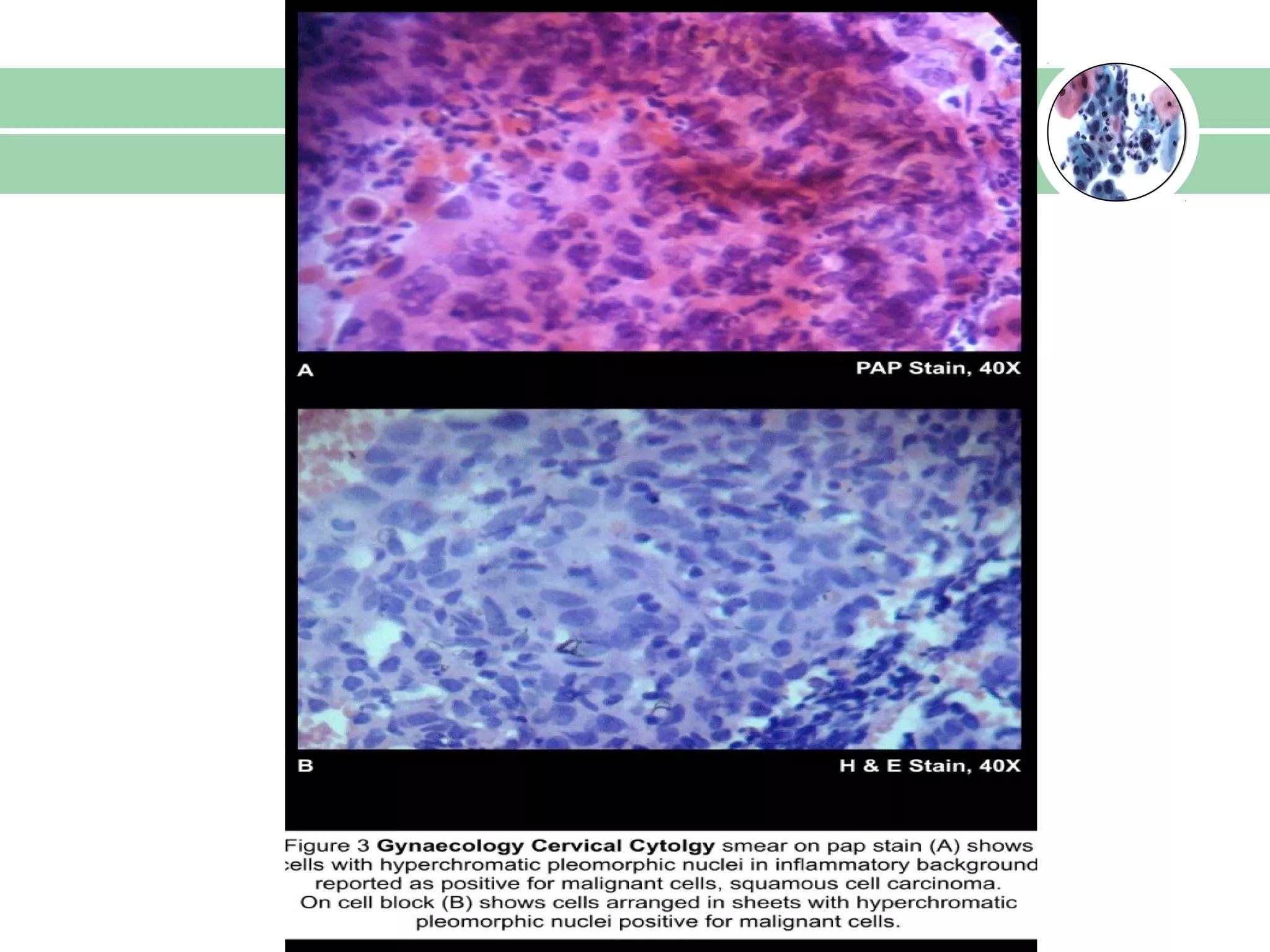
Cell blocks provide diagnostic information in addition to regular cytology slides. They allow examination of histological structure and use of ancillary tests like special stains and immunohistochemistry. A cell block is prepared by concentrating cells from cytology specimens using various methods like centrifugation or thrombin clotting. This allows cells to be processed and examined like histology samples. Cell blocks improve diagnostic accuracy for body fluids and fineneedle aspiration samples. They are useful for identifying primary tumor sites, distinguishing reactive from malignant cells, and enabling molecular testing.