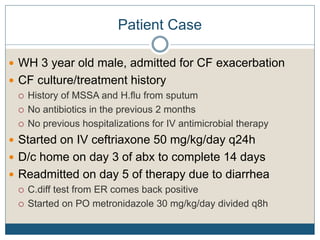
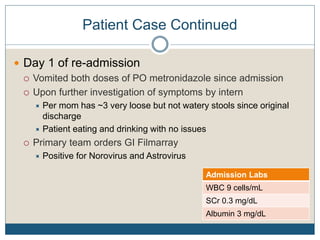
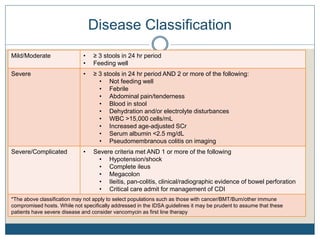
1) The document discusses guidelines for treating Clostridium difficile (C. diff) infection in children, including risk factors, disease classification, and treatment recommendations.
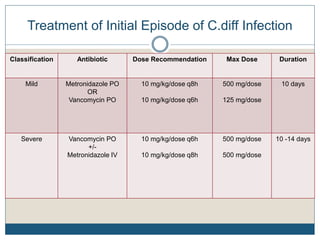
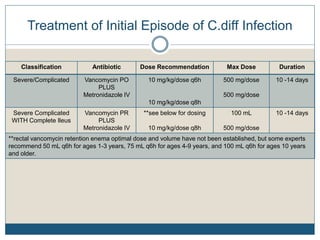
2) Treatment for initial mild or moderate C. diff includes oral metronidazole or vancomycin for 10 days, while severe cases recommend oral vancomycin with or without IV metronidazole for 10-14 days.
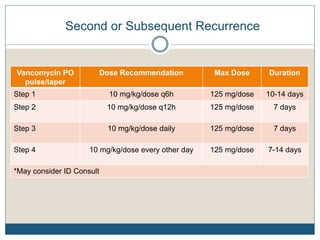
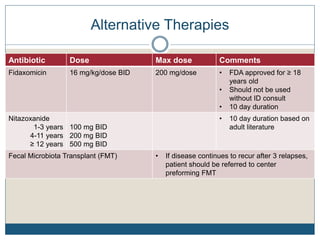
3) Recurrent cases suggest pulsed oral vancomycin dosing or alternative therapies like fidaxomicin or nitazoxanide under infectious disease guidance.






![Clinical Pearls For Treatment - Metronidazole
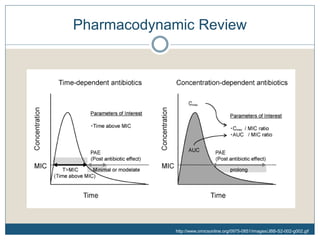
Bioavailability >90%
Distributes widely throughout the body
Metabolized into multiple metabolites (1 active)
Active metabolite has a longer half-life than parent compound
Half-life of parent compound + metabolite = 6-10 hrs
Concentration-dependent antibiotic
Post antibiotic effect around 3 hrs
Lamp KC, et al. Clin Pharmacokinet 1999;36(5):353-373.
Metronidazole [prescribing information] Sellersville, PA: Teva; 2011.](https://image.slidesharecdn.com/5-whatsnewinc-190816132511/85/What-s-new-in-c-diff-15-320.jpg)
![Clinical Pearls For Treatment - Metronidazole
Commercially available liquid product
Grape flavored
Important counseling points
Recommended to take with food to minimize GI effects
Disulfuram-like reaction with alcohol
Cumulative neurotoxicity with repeated exposures
Lamp KC, et al. Clin Pharmacokinet 1999;36(5):353-373.
Metronidazole [prescribing information] Sellersville, PA: Teva; 2011.](https://image.slidesharecdn.com/5-whatsnewinc-190816132511/85/What-s-new-in-c-diff-17-320.jpg)