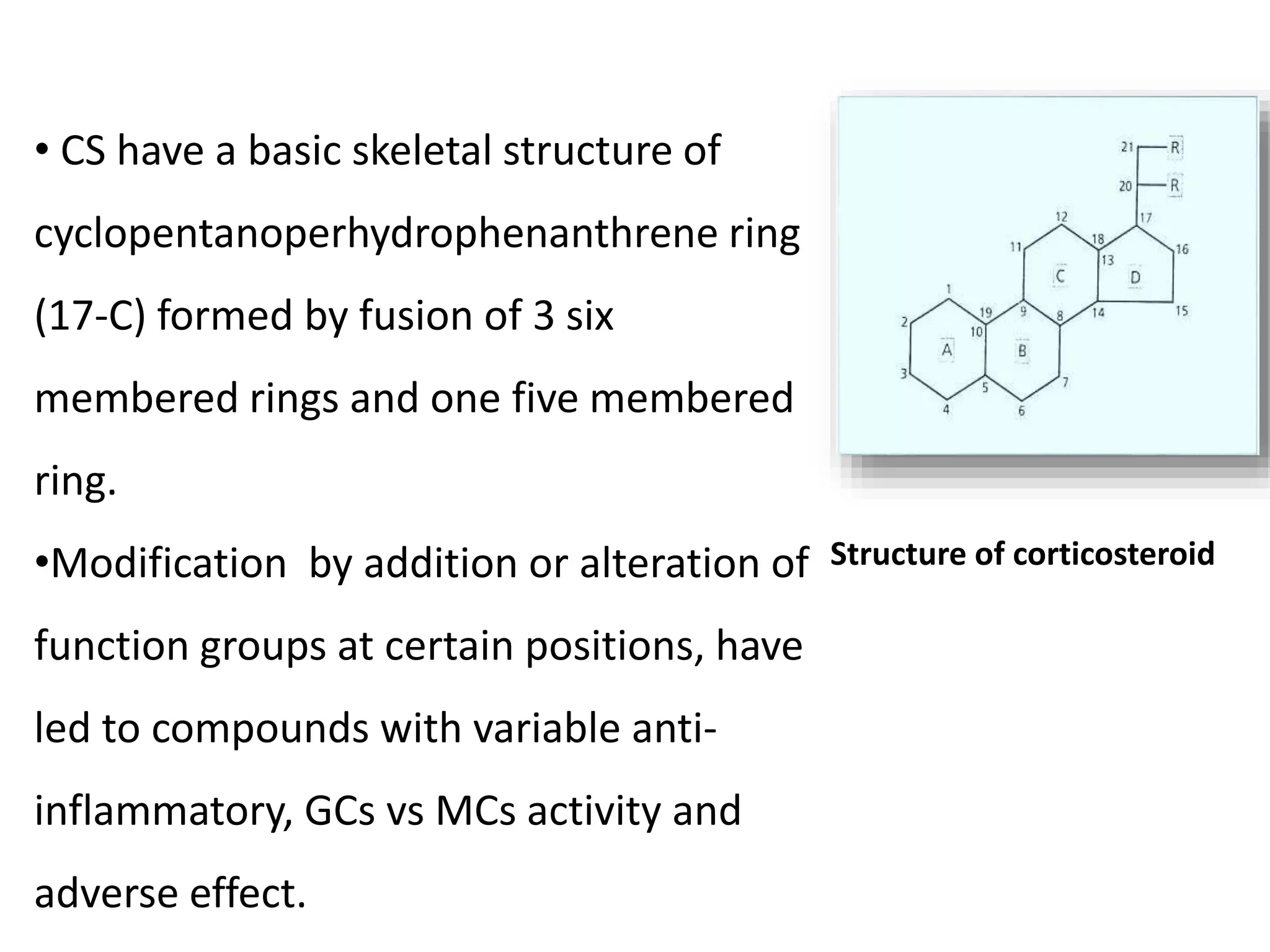
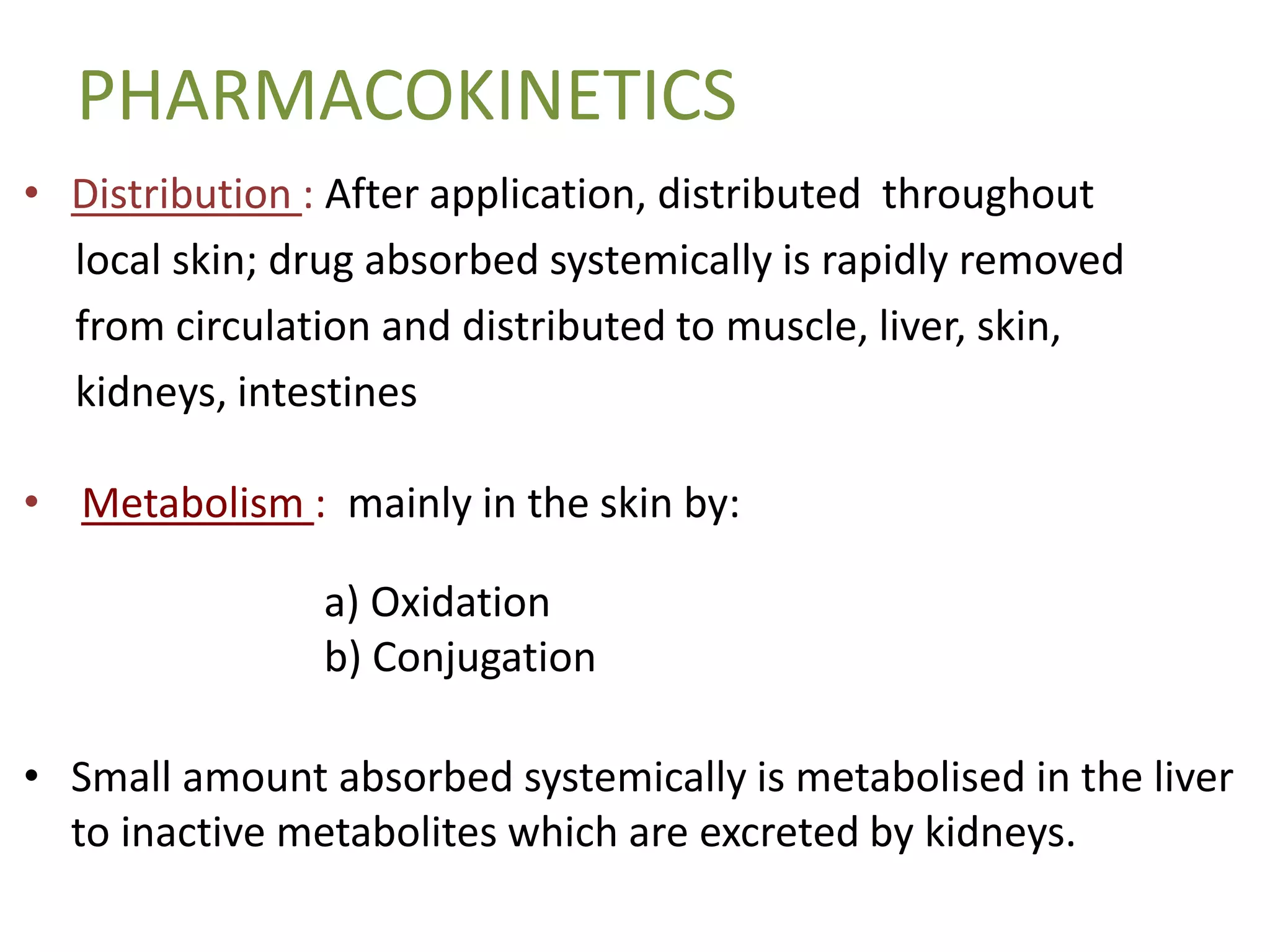
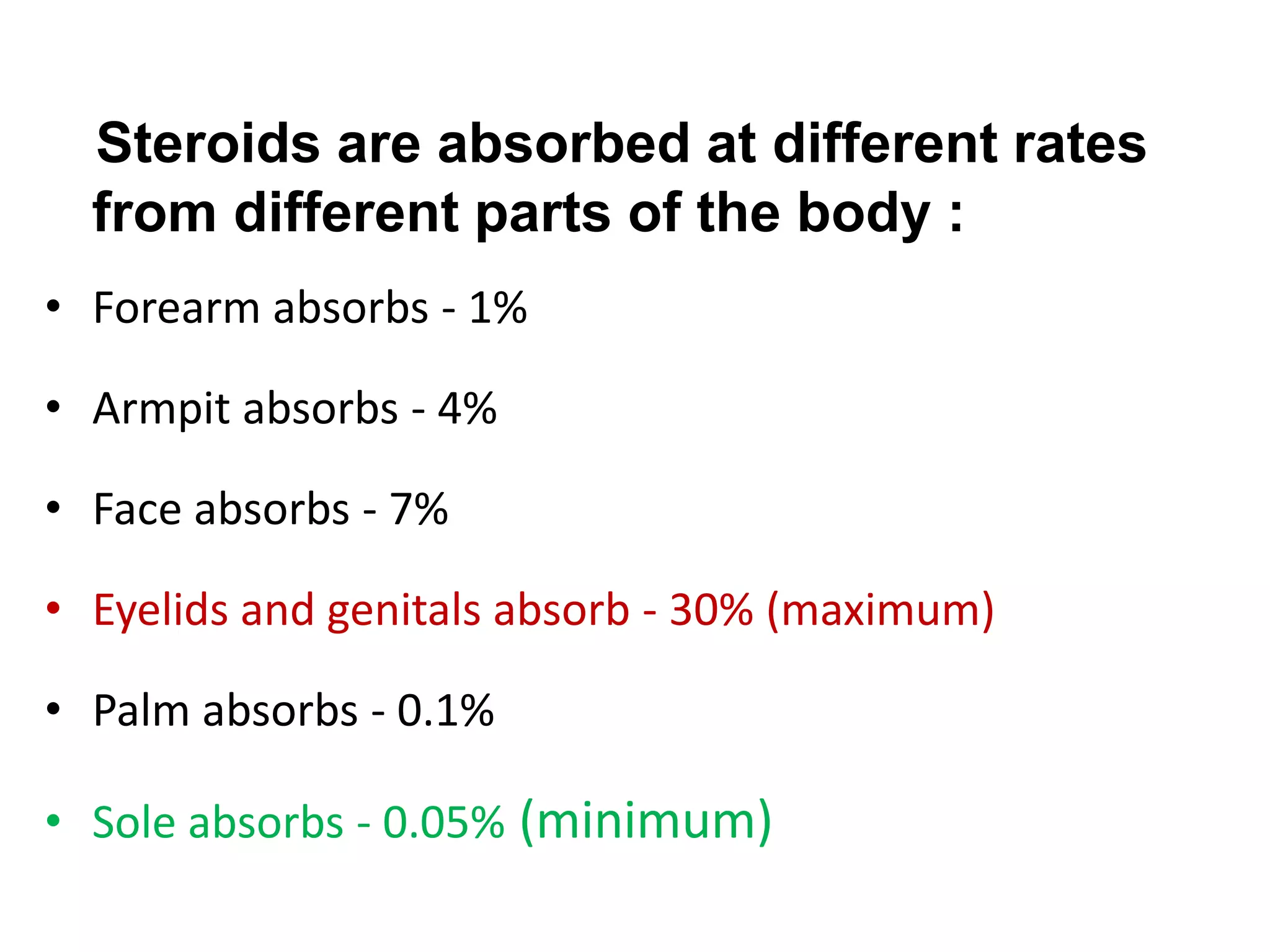
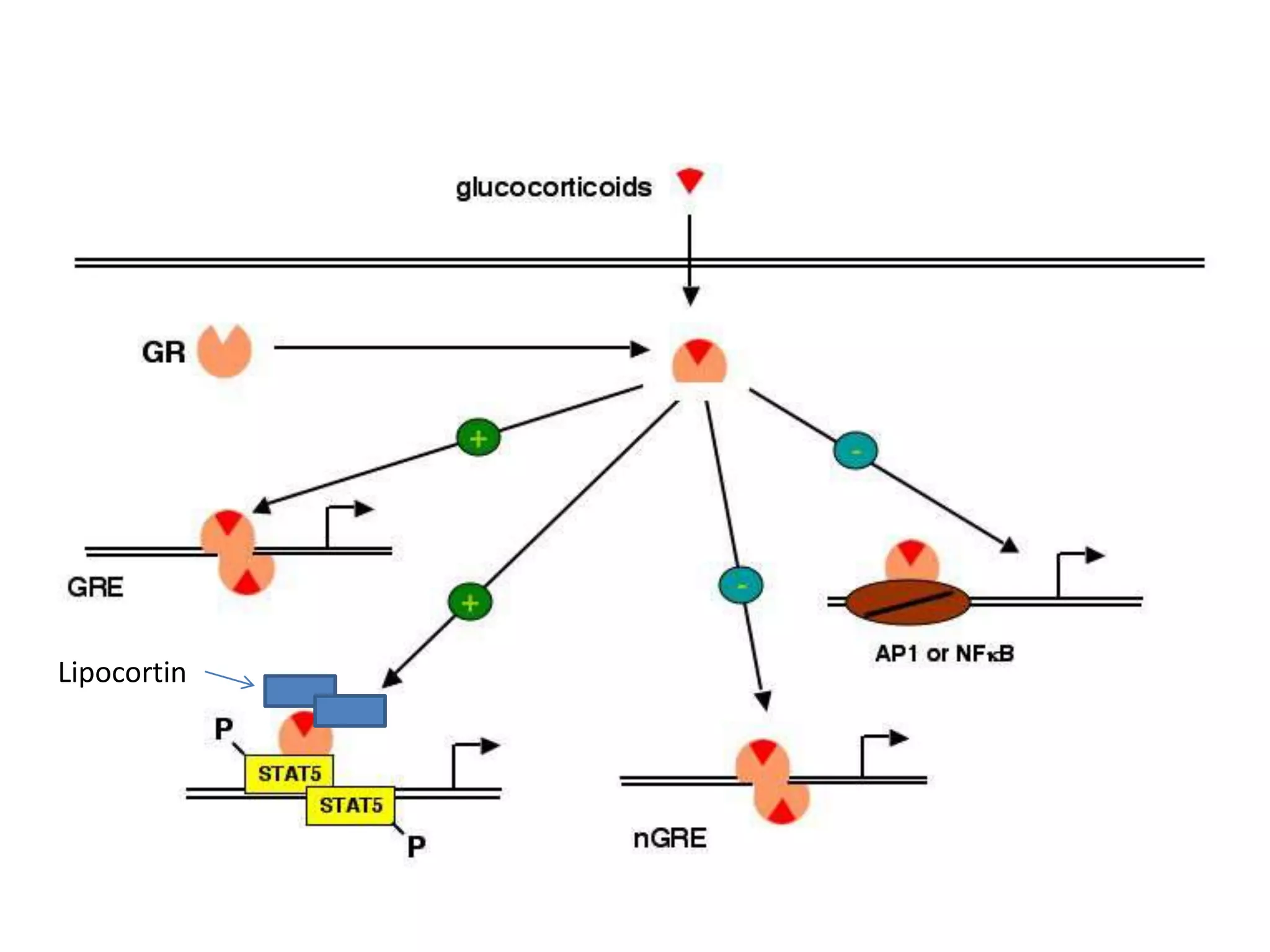
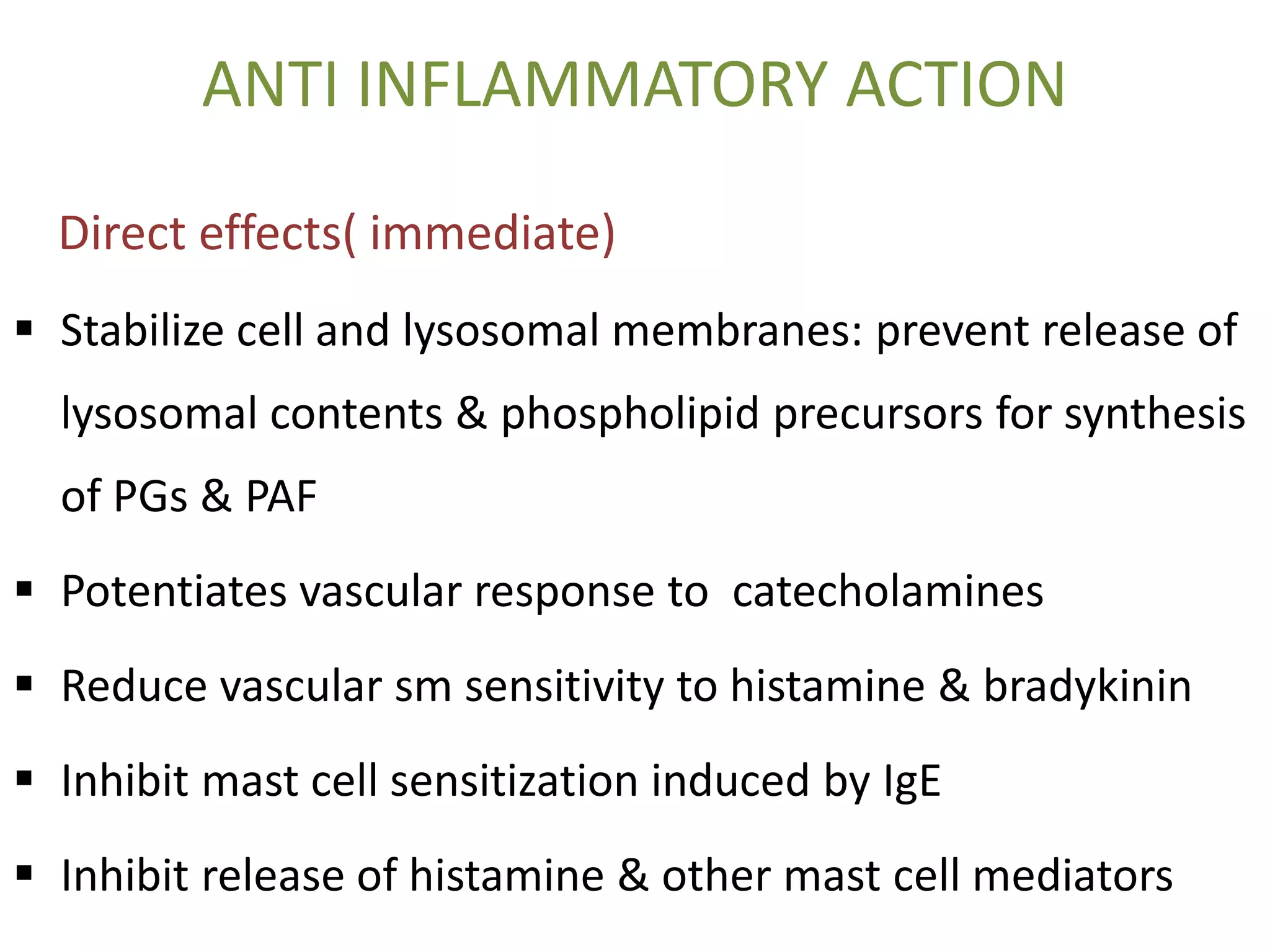
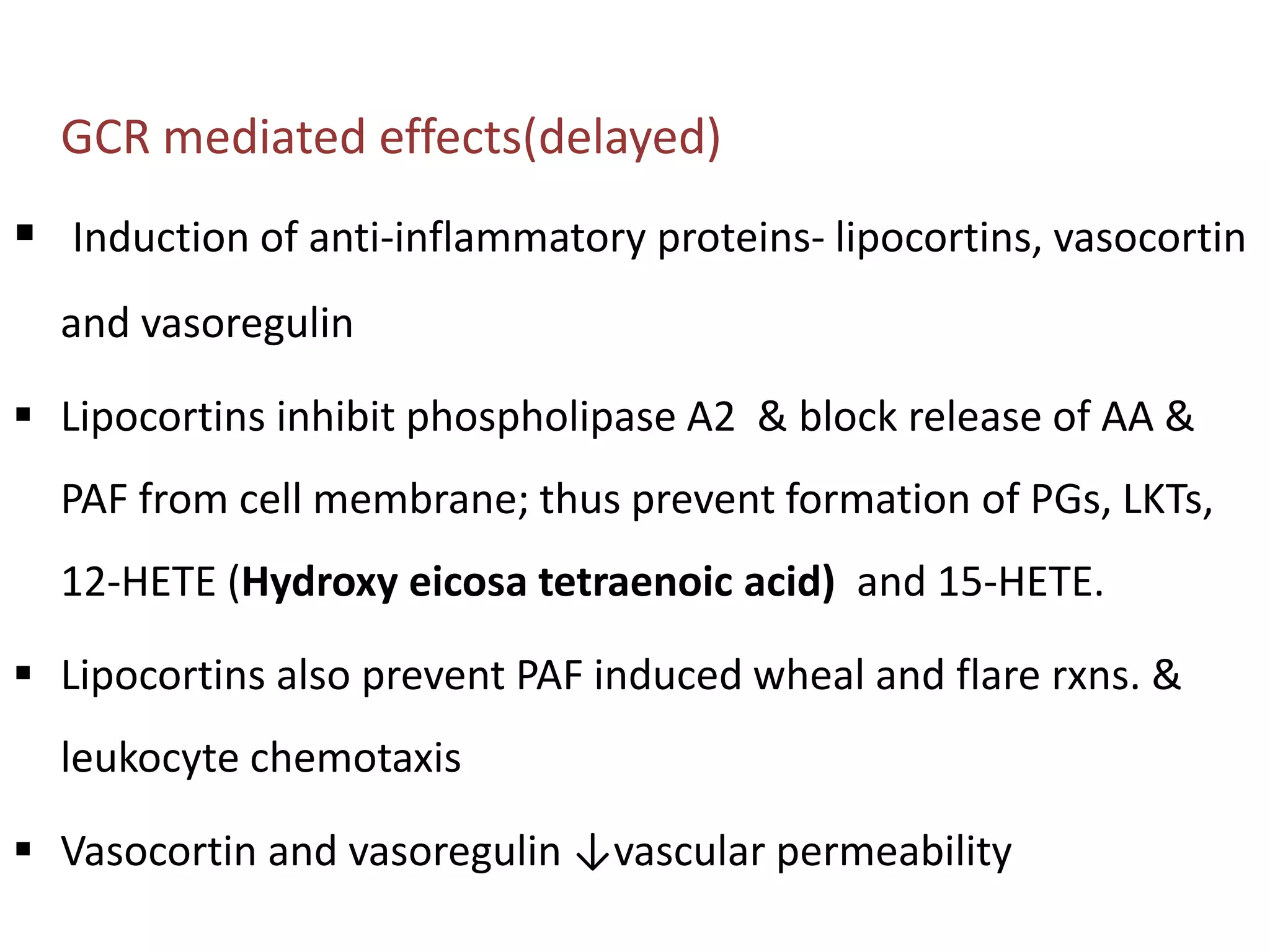
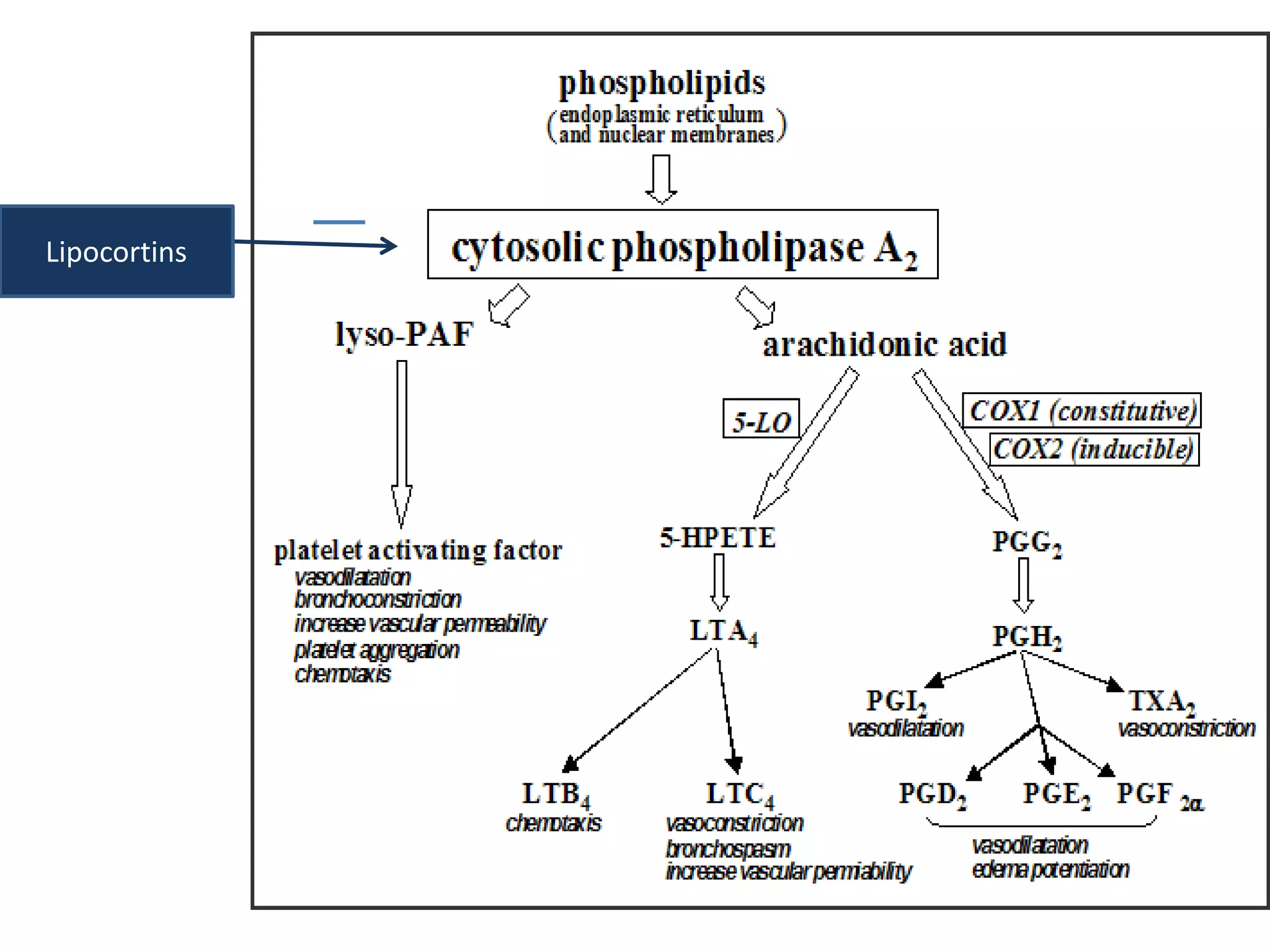
This document discusses topical corticosteroids (TCS). It begins by describing the discovery and structure of corticosteroids. It then covers the pharmacokinetics of TCS, noting they are distributed in the skin and absorbed systemically before being metabolized in the liver. The potency of a TCS preparation depends on its structure, vehicle, and skin condition. The document outlines the anti-inflammatory, antiproliferative, and atrophogenic mechanisms of action of TCS. It concludes by listing common indications and side effects of TCS.