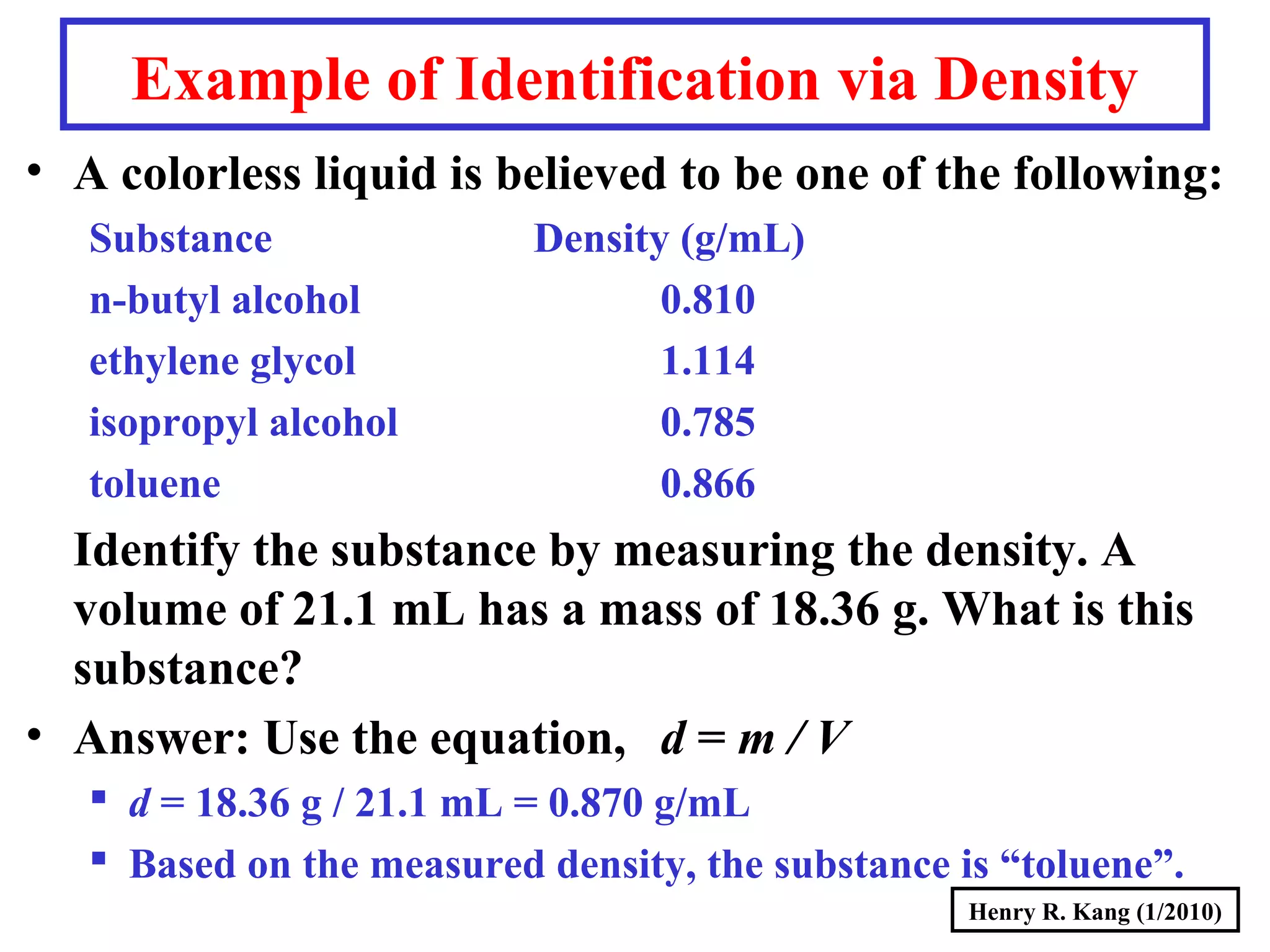
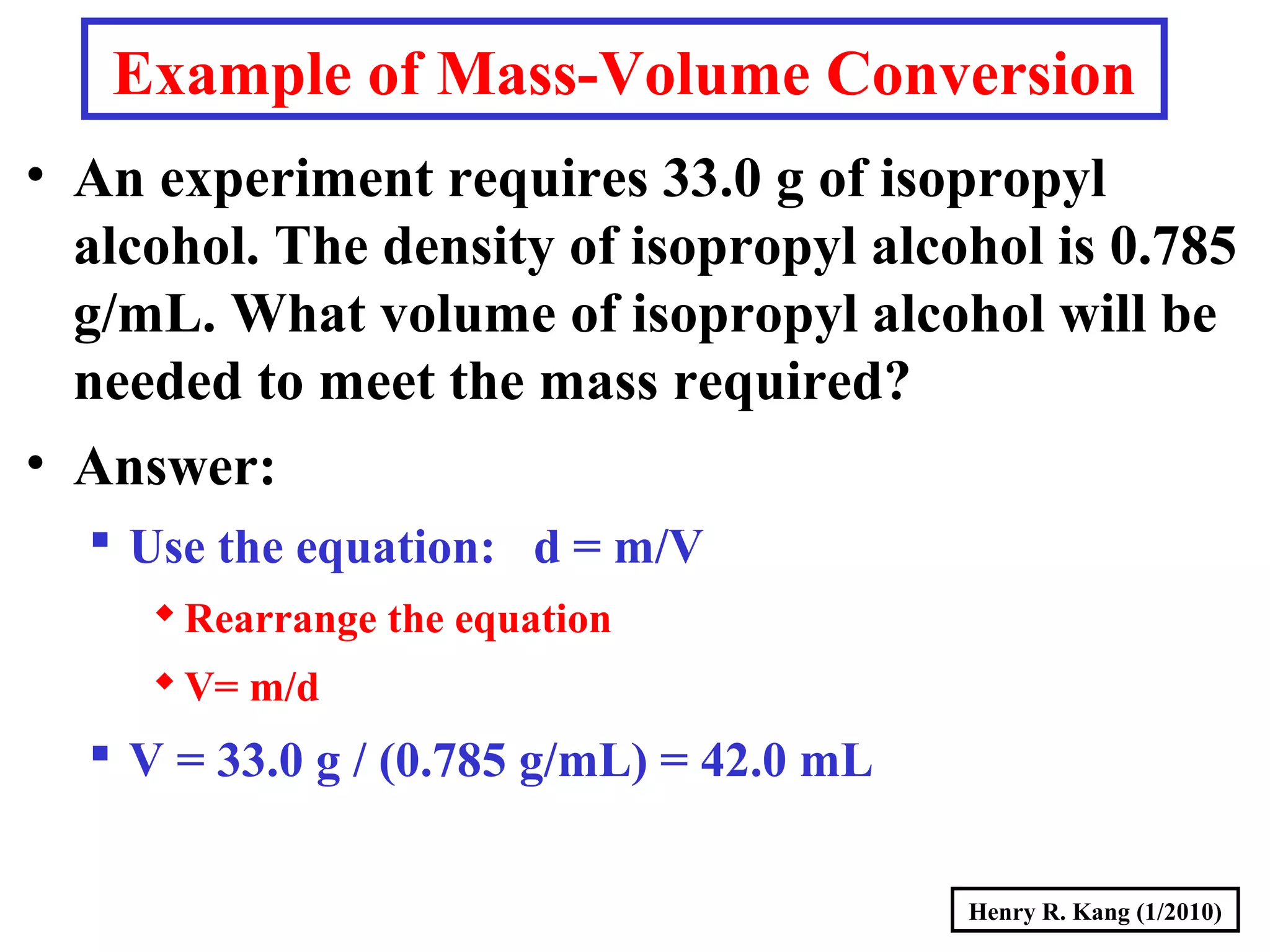
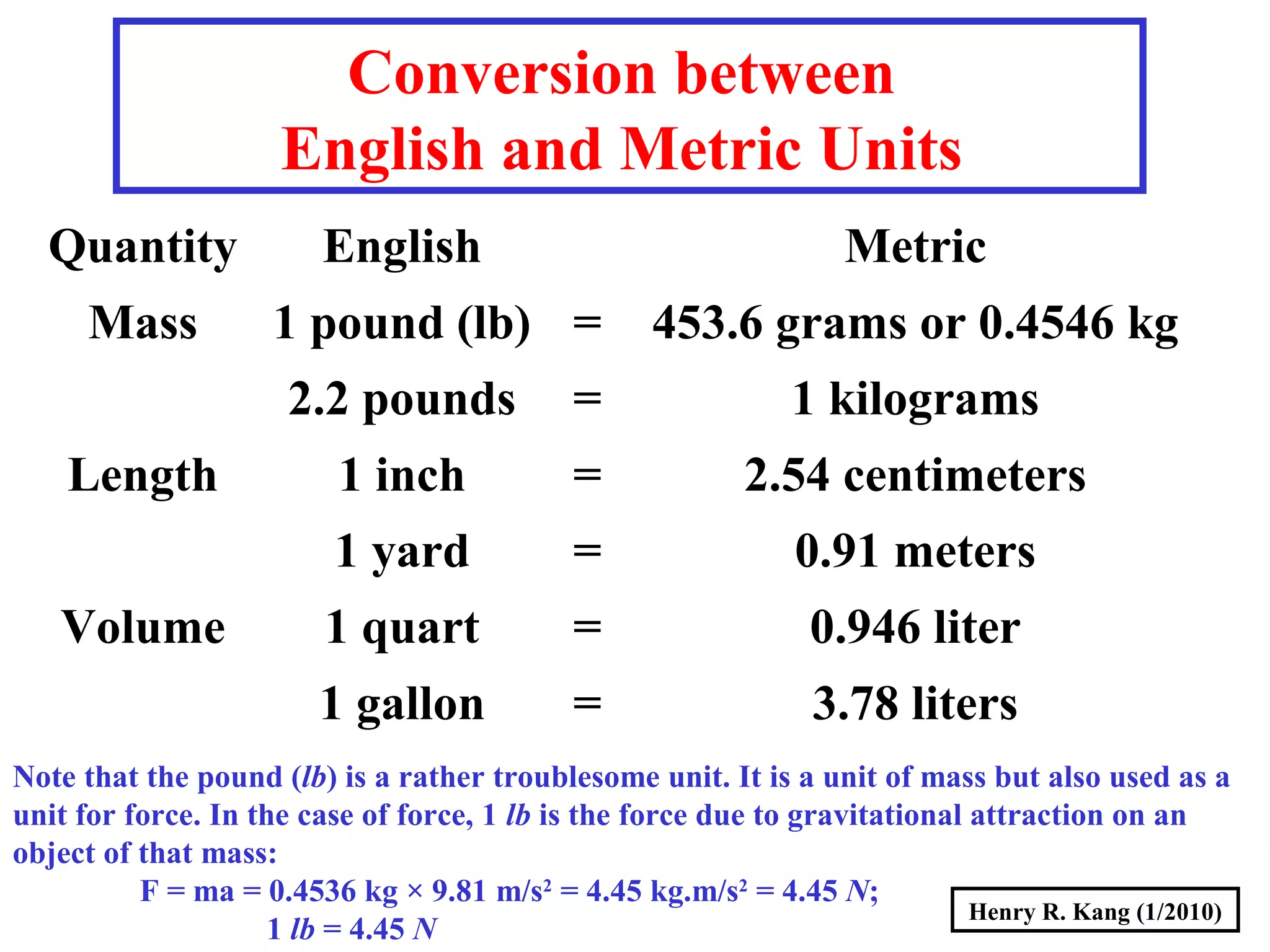
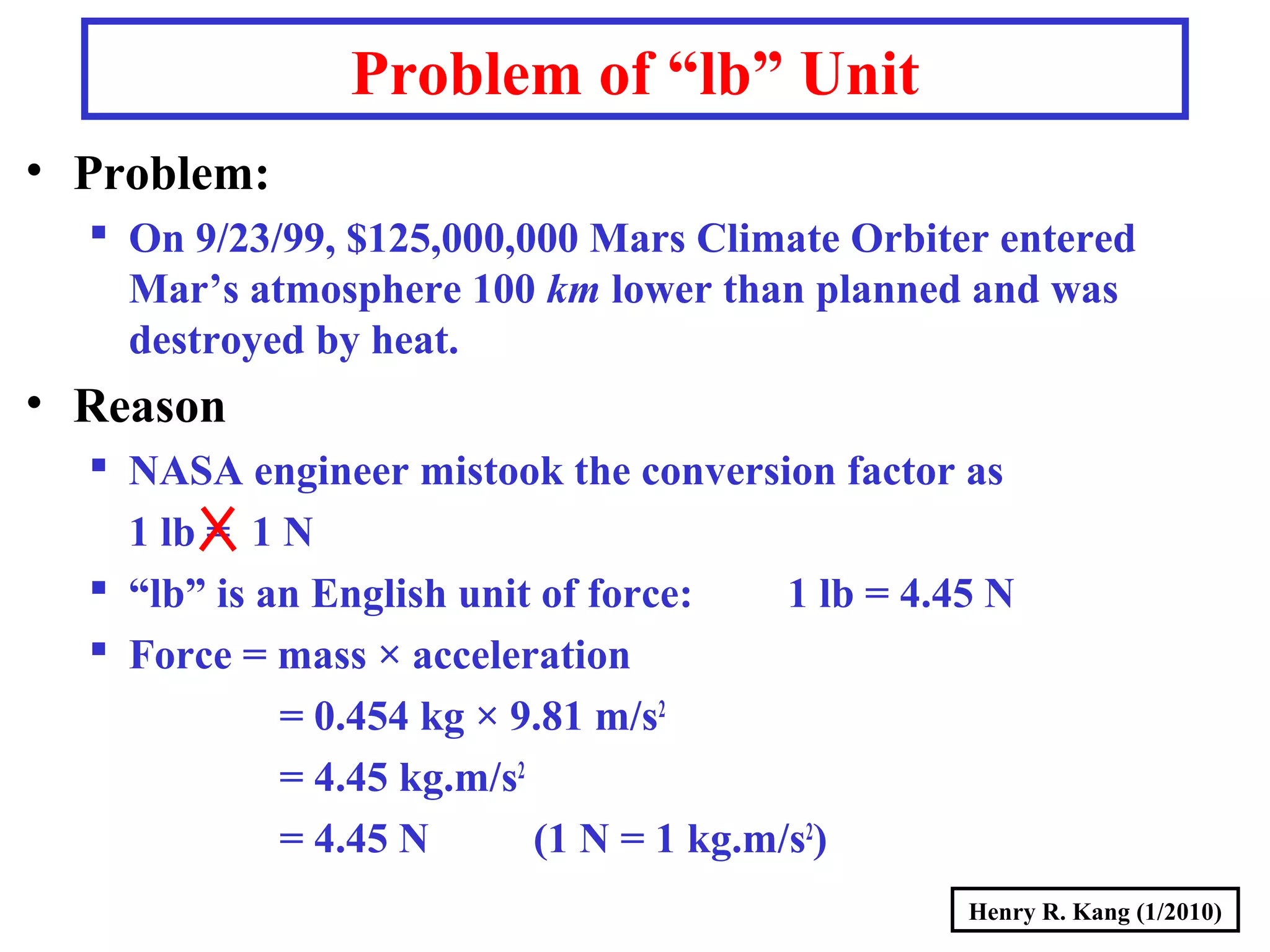
This document summarizes key concepts about measurement from a general chemistry lecture, including:
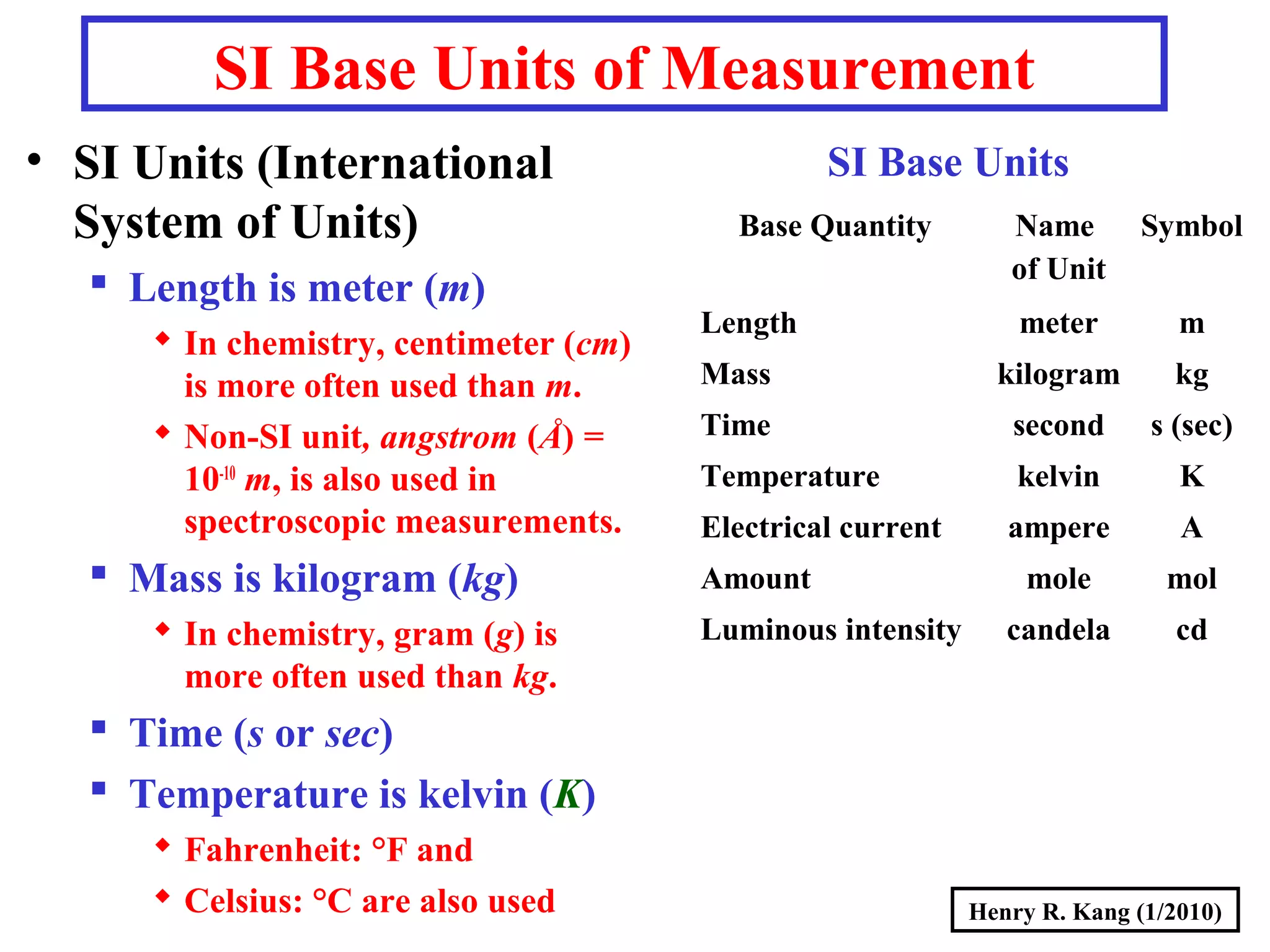
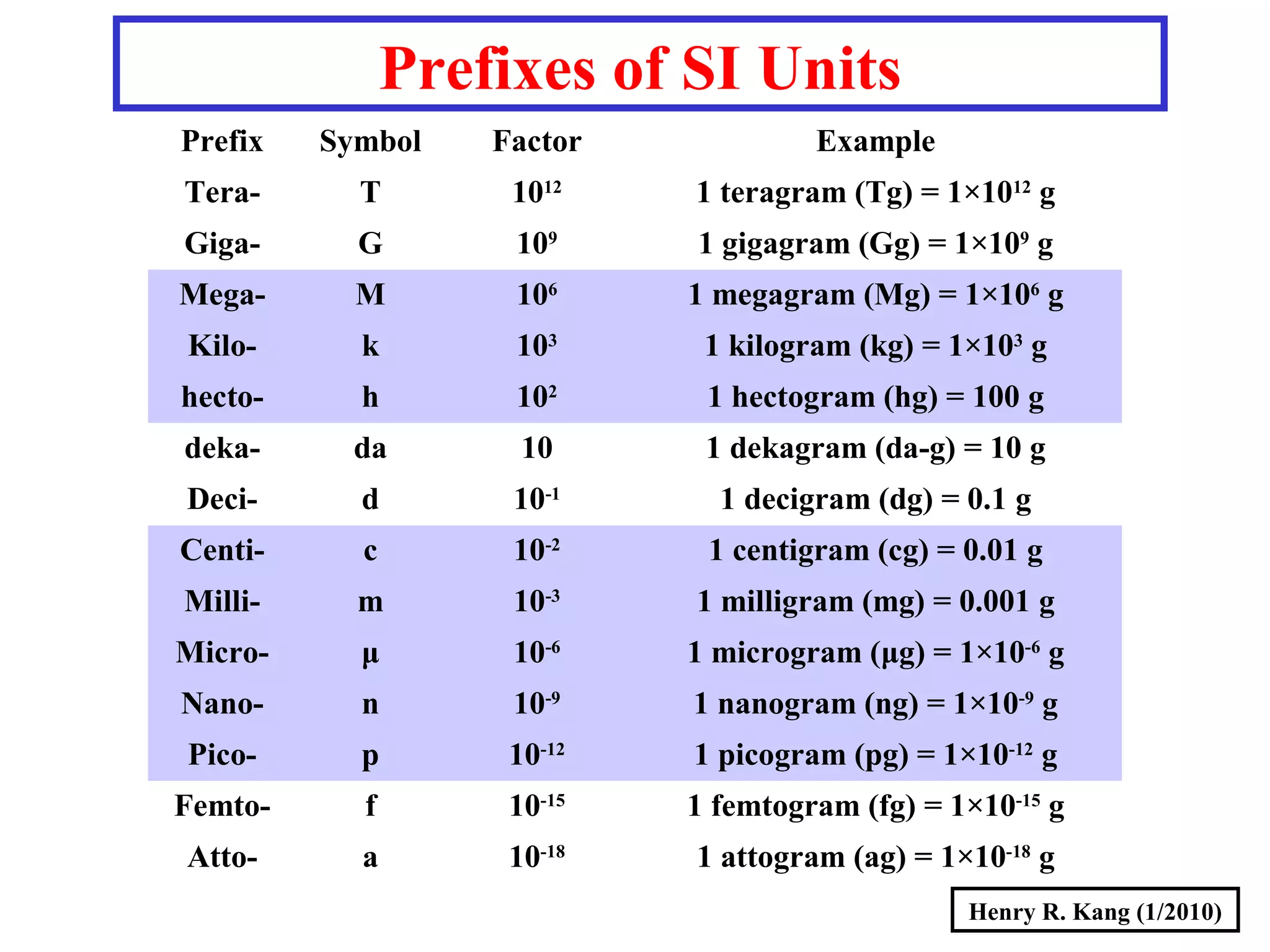
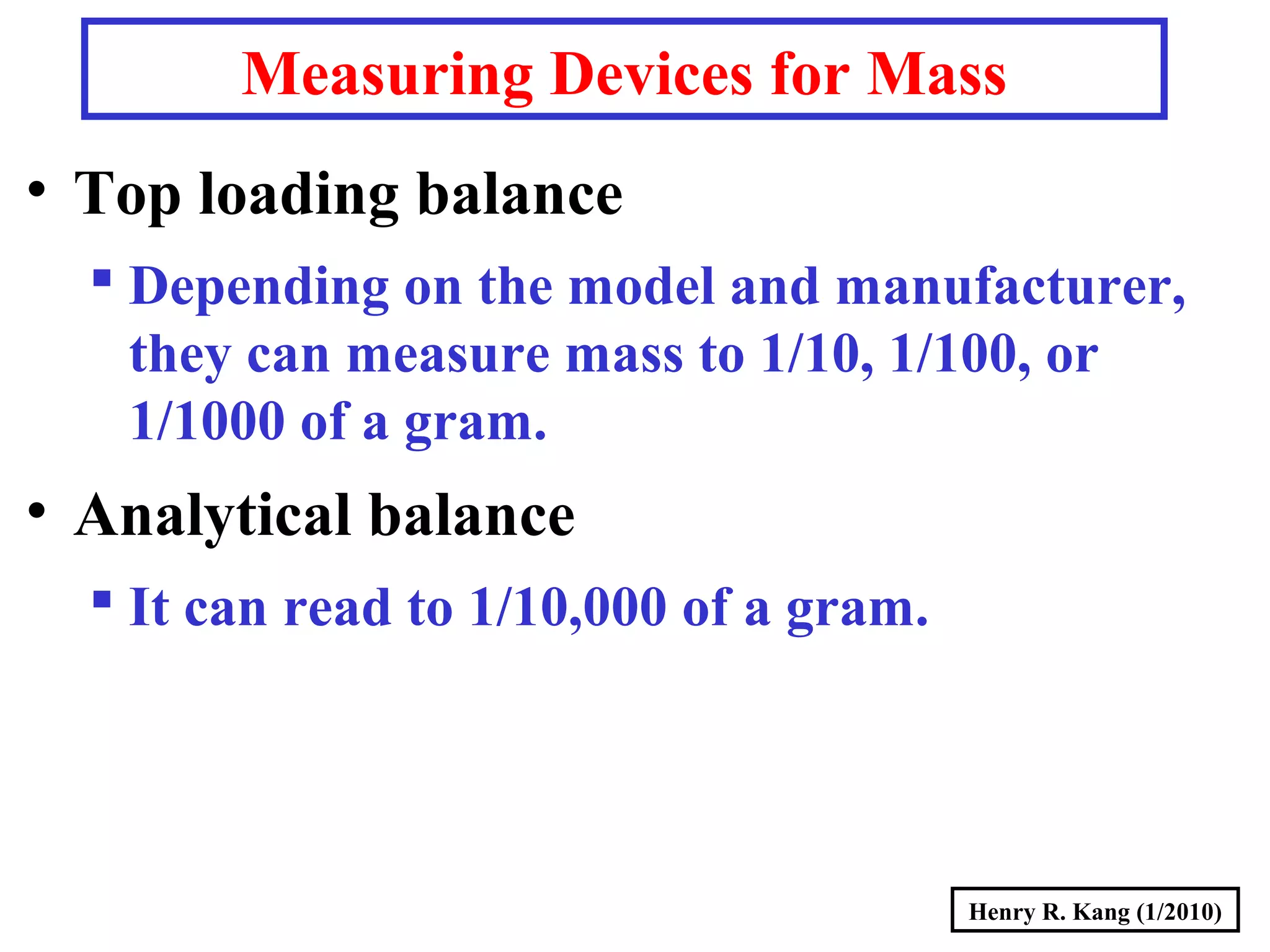
- SI base units for length, mass, time, temperature and others. Prefixes are used to modify the units.
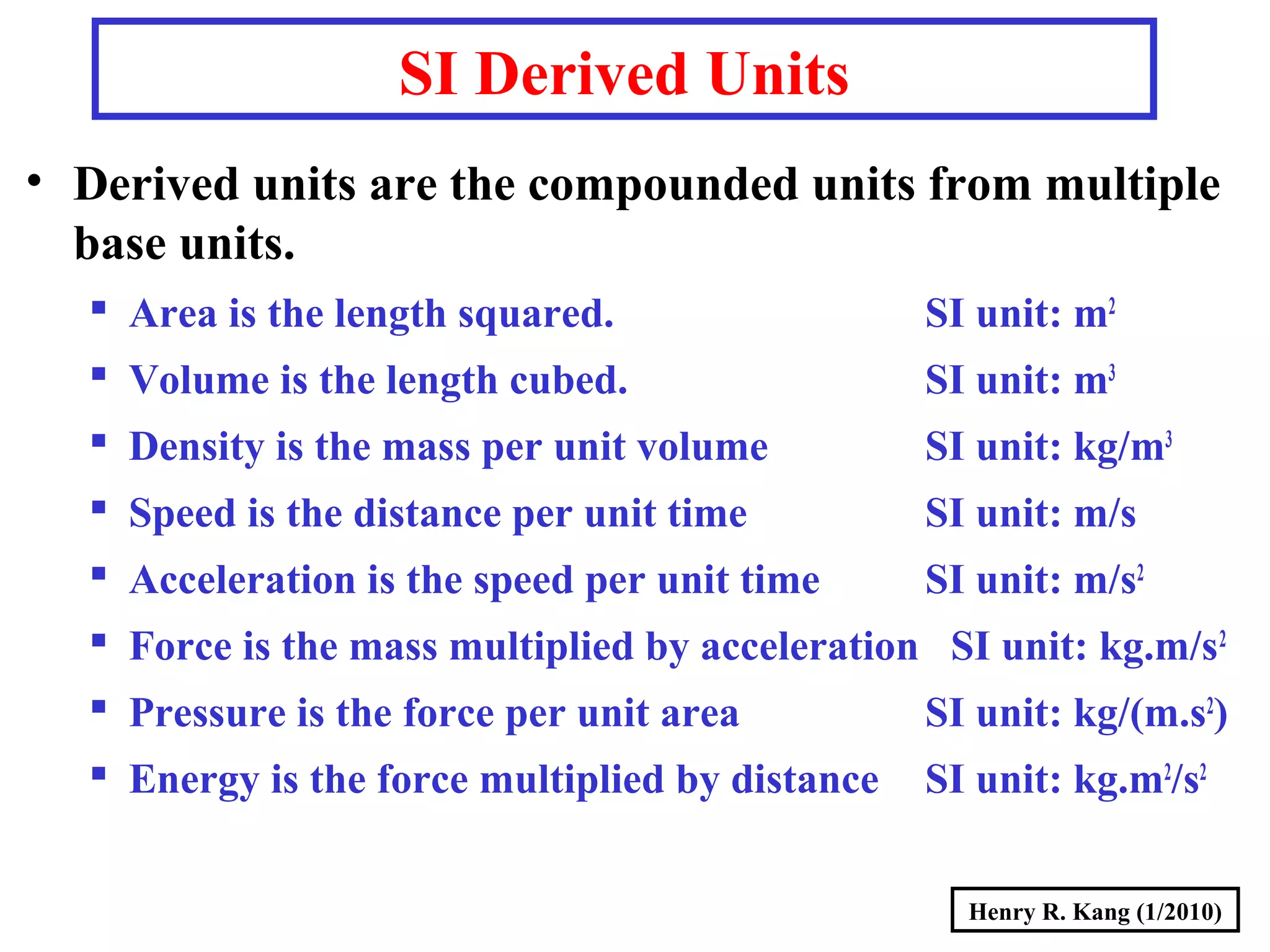
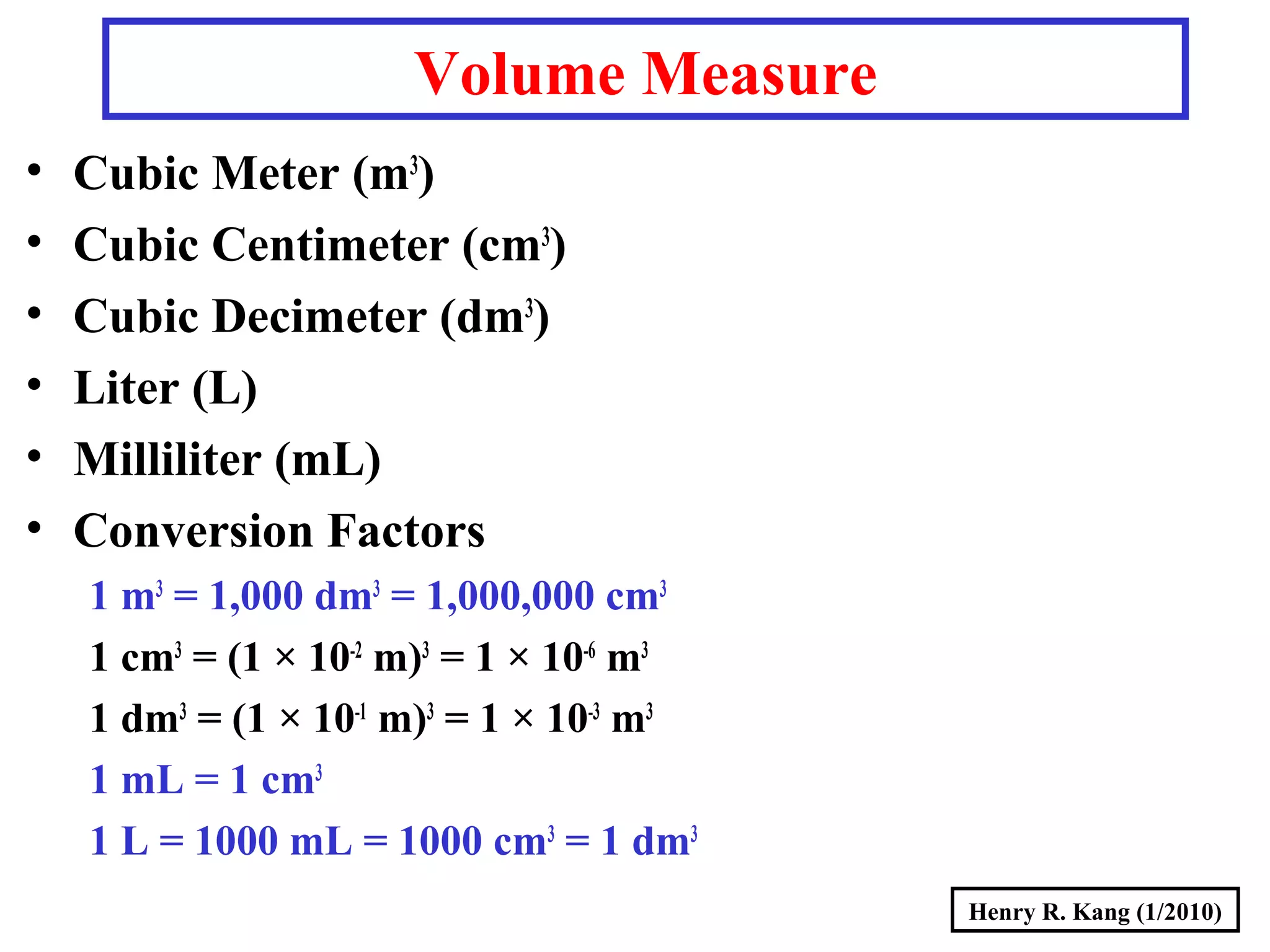
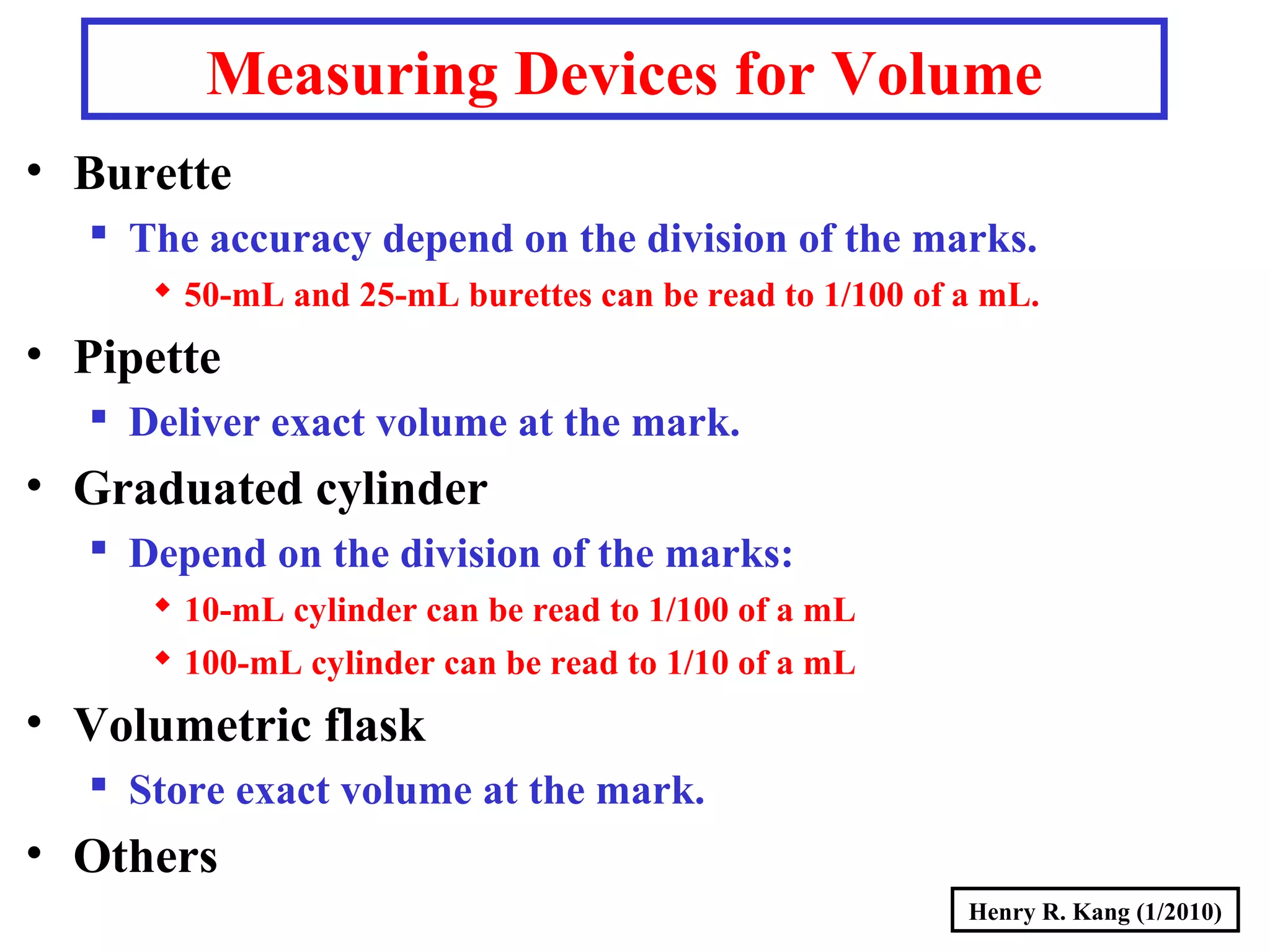
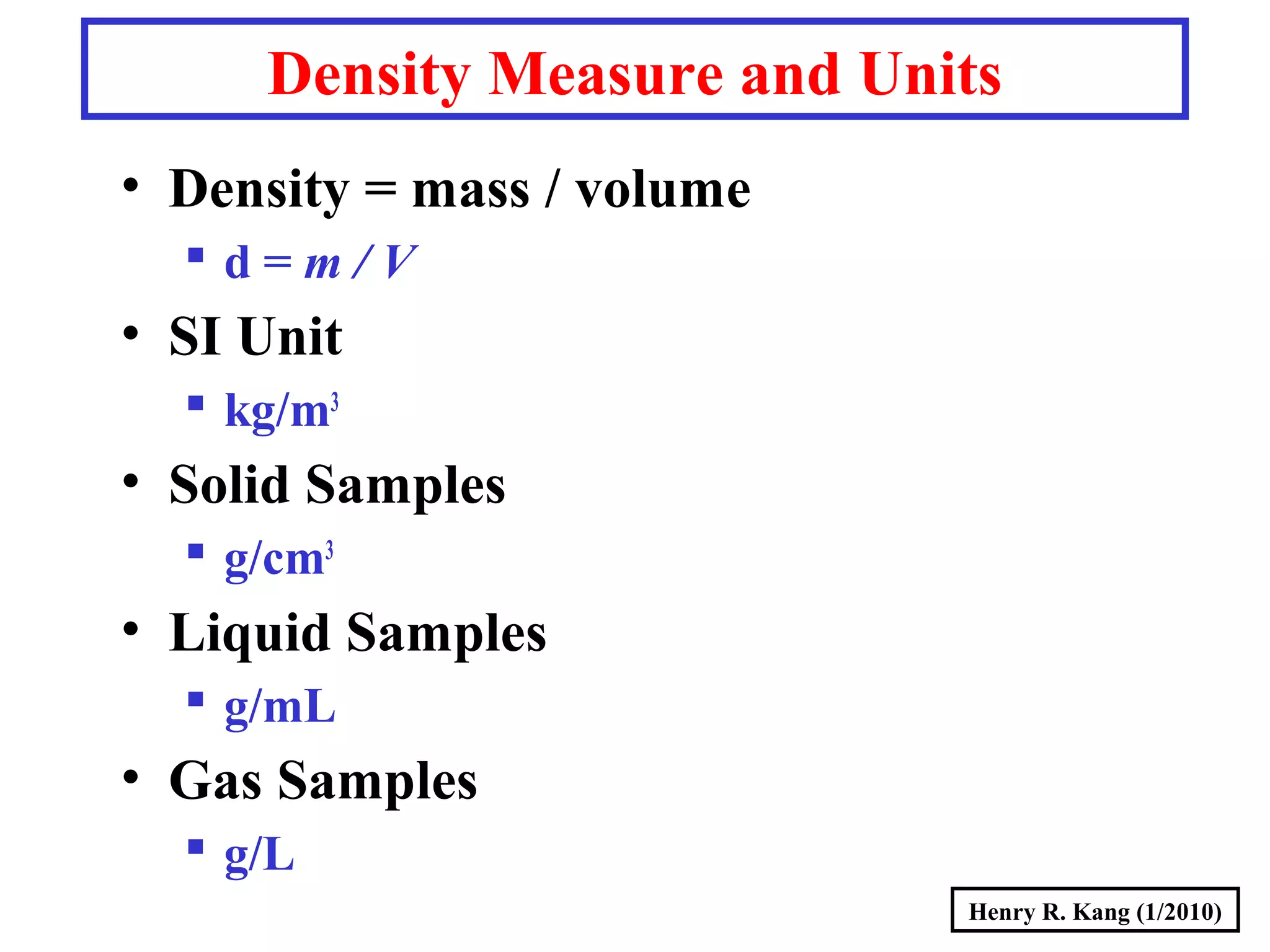
- Derived SI units are composed from multiple base units, like area, volume, density and more.
- English units are also discussed and converted to metric units.
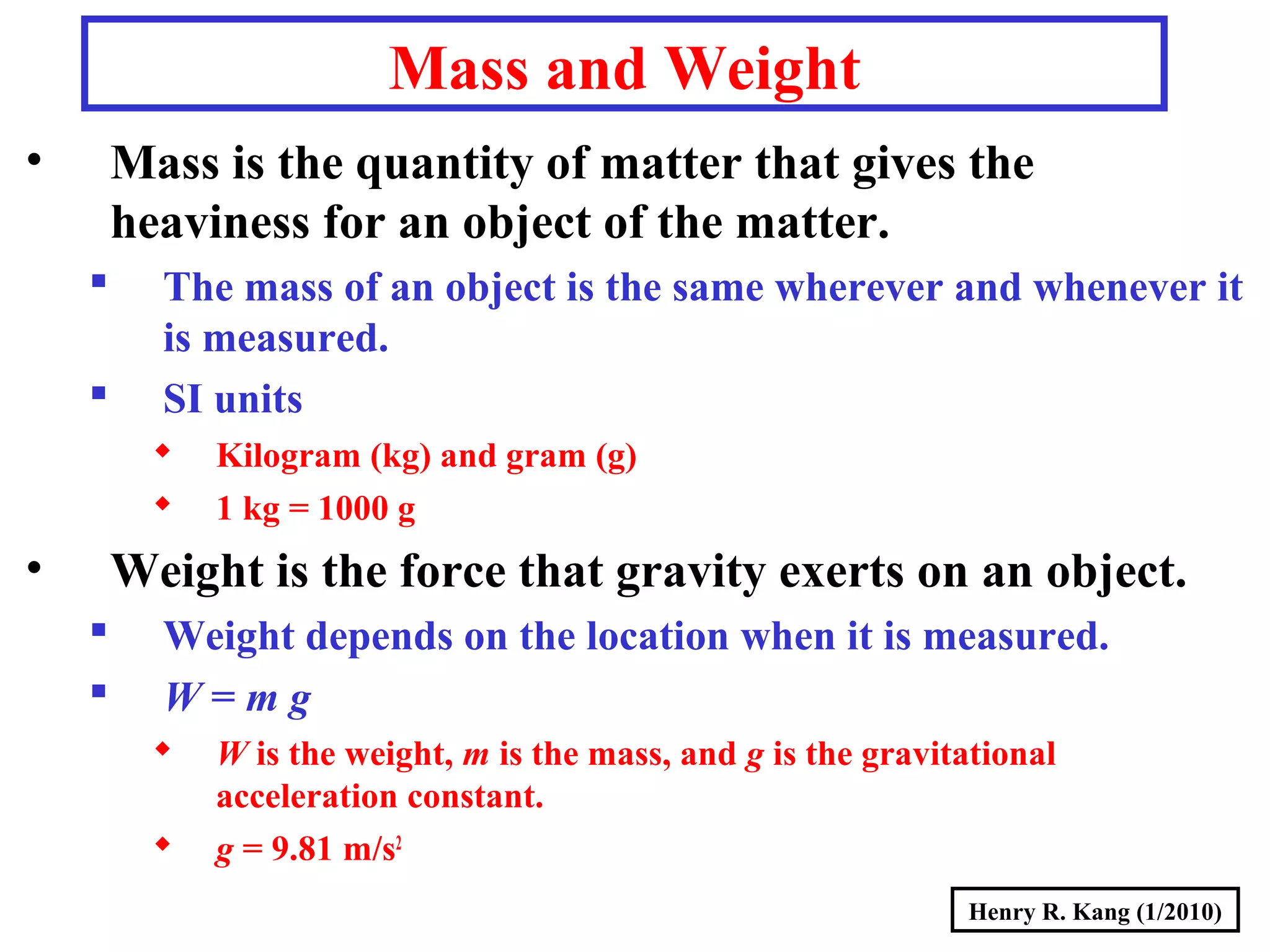
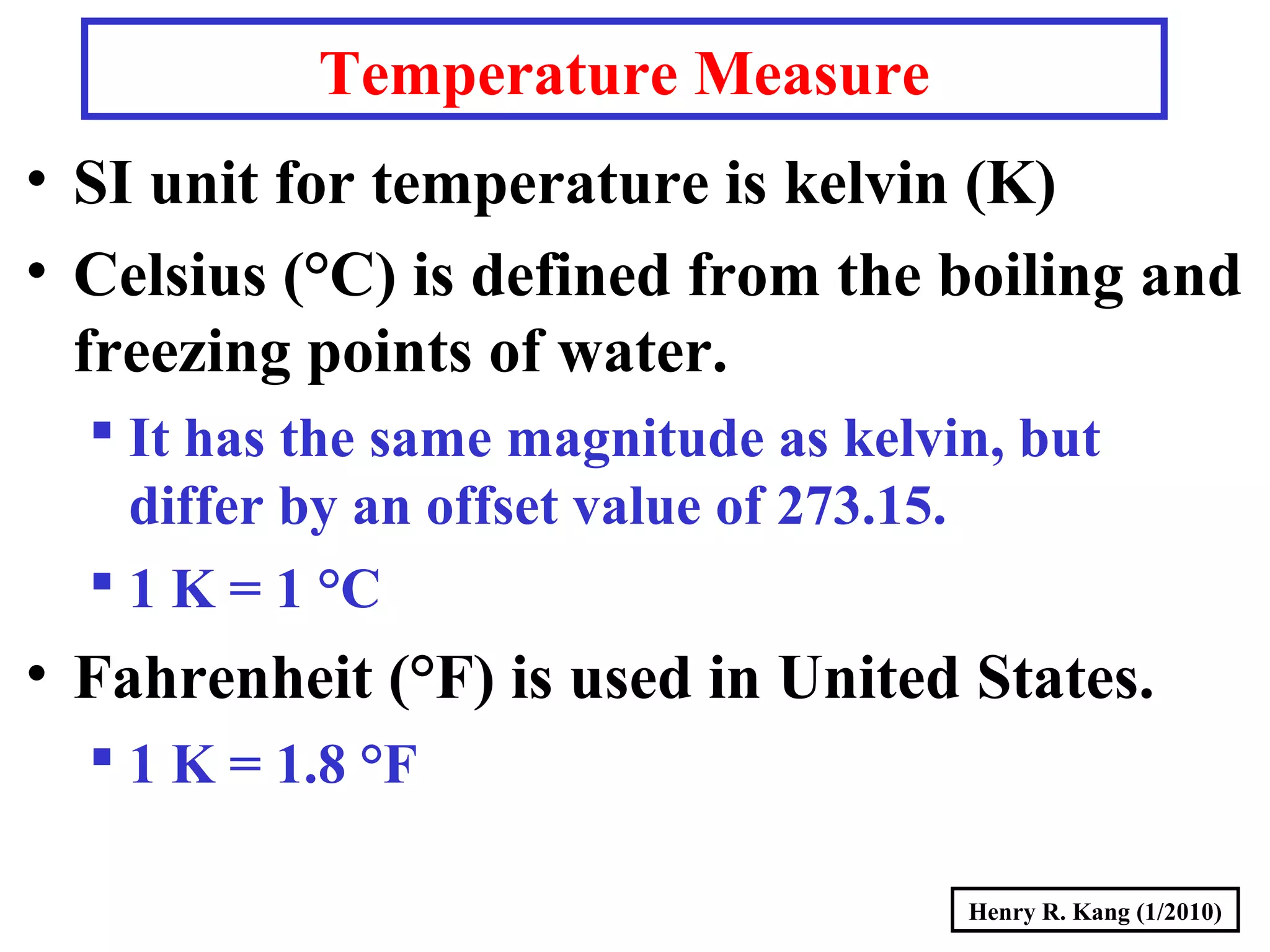
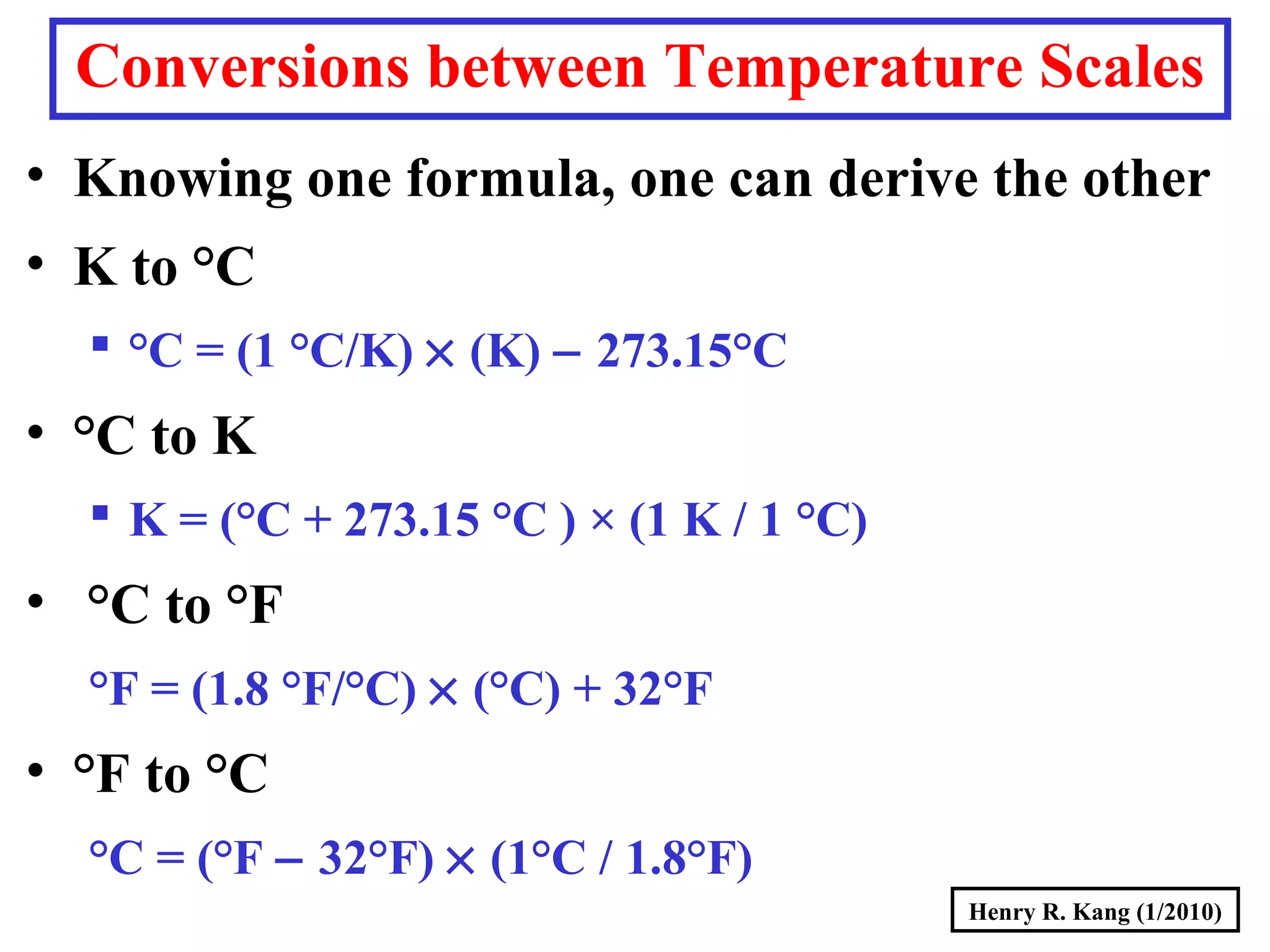
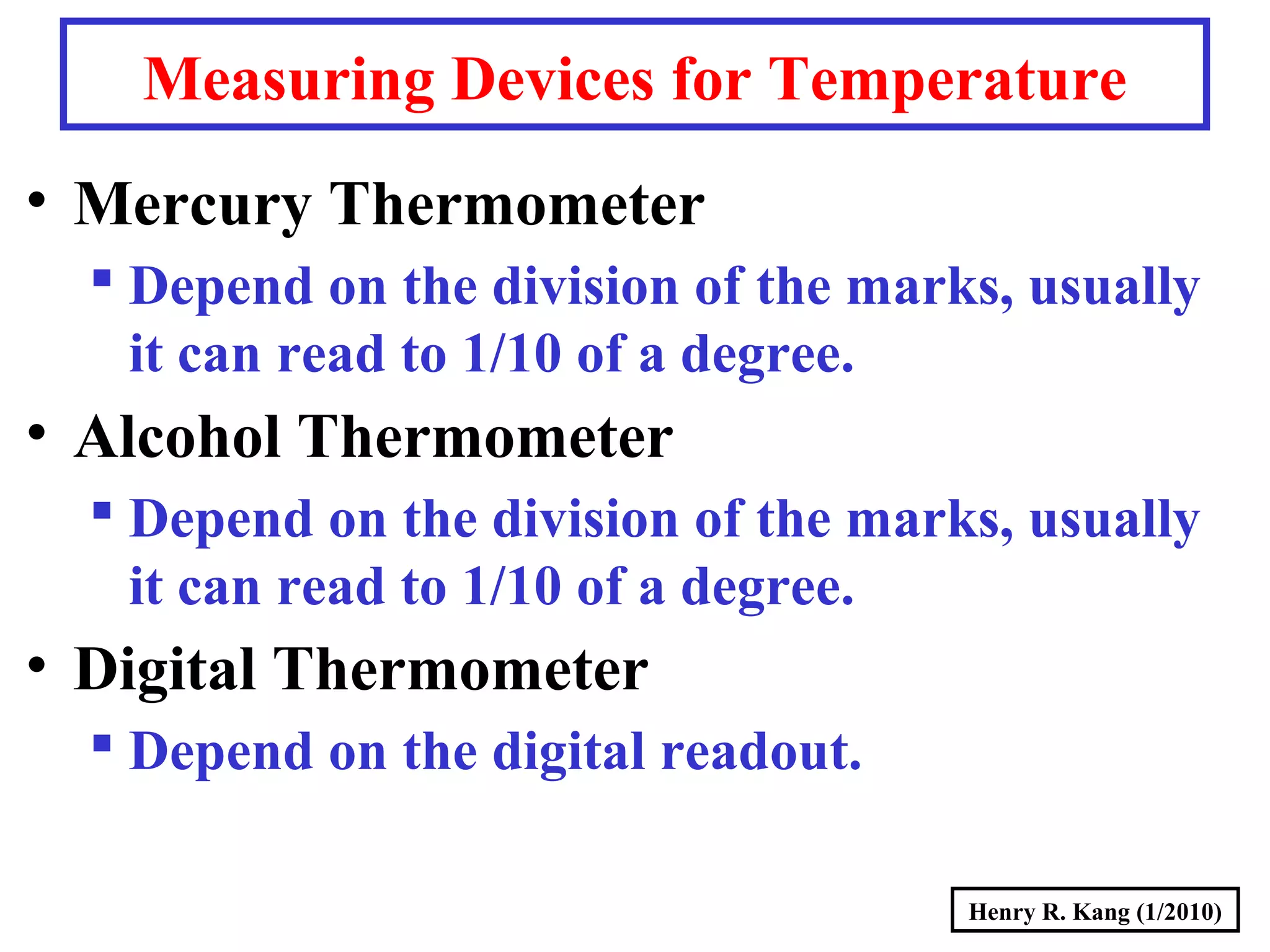
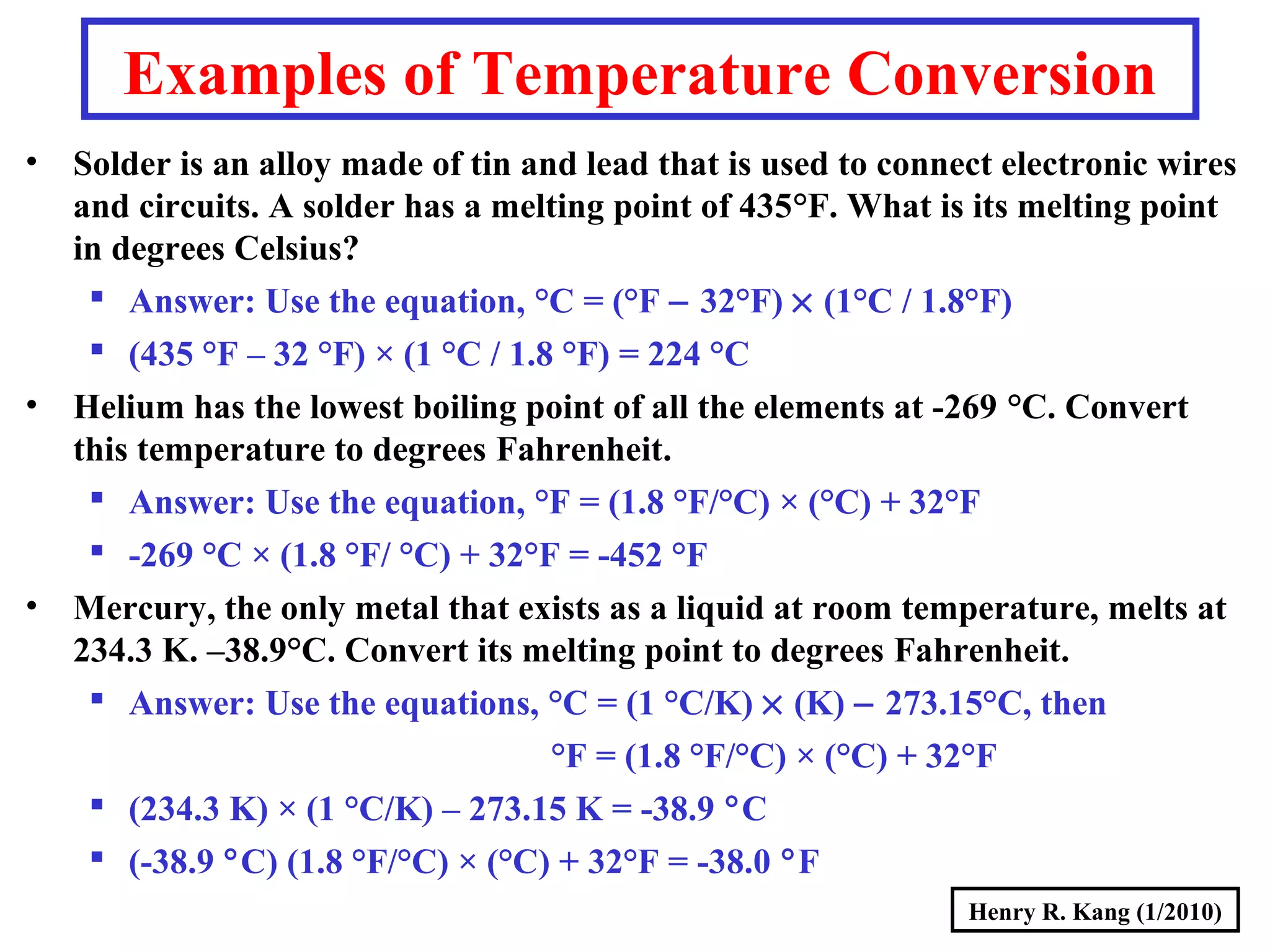
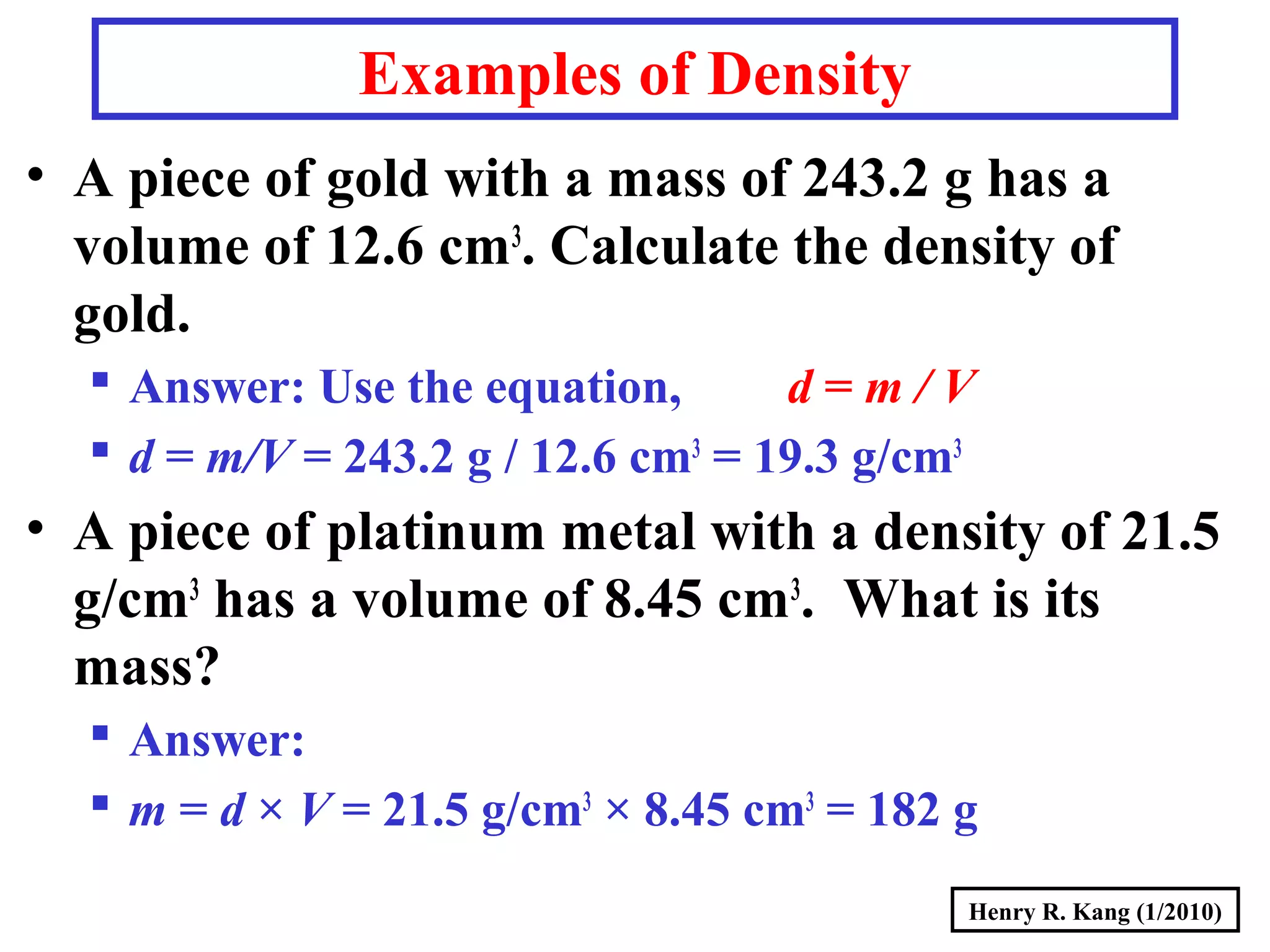
- Concepts like mass vs weight, temperature scales, density, and the problematic "lb" unit are explained. Examples are provided to demonstrate calculations and conversions between units.