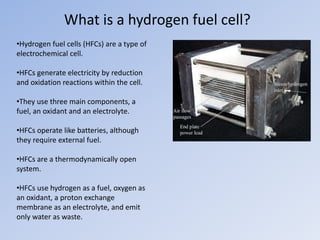
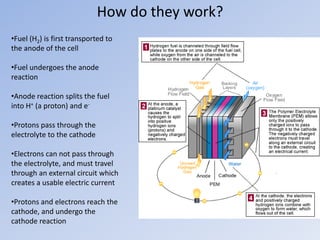
Hydrogen fuel cells generate electricity through electrochemical reactions between hydrogen and oxygen. They have three main components - a fuel (hydrogen), oxidant (oxygen), and electrolyte. Hydrogen fuel cells split hydrogen into protons and electrons at the anode, and then recombine them with oxygen at the cathode to produce water and energy. While fuel cells can power vehicles and electronics, challenges remain around developing hydrogen infrastructure and reducing costs to levels comparable with gasoline vehicles.