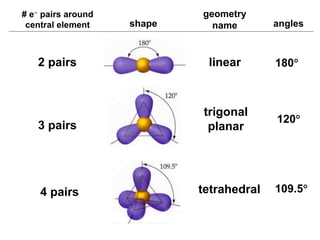
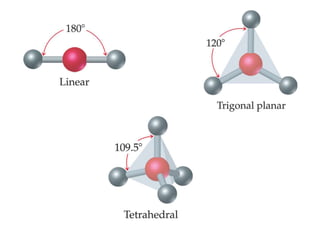
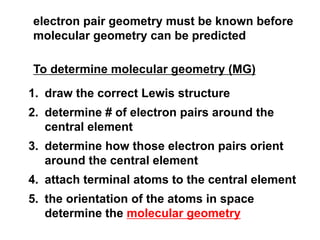
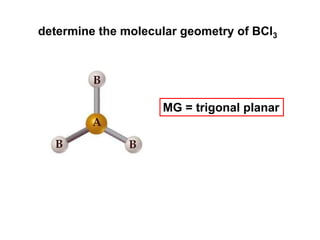
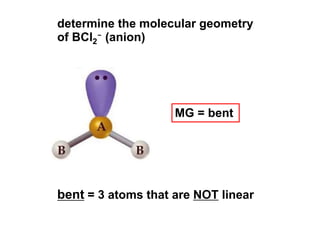
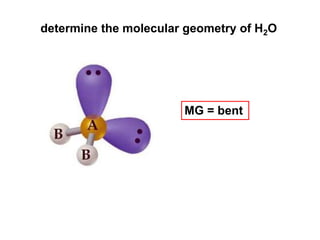
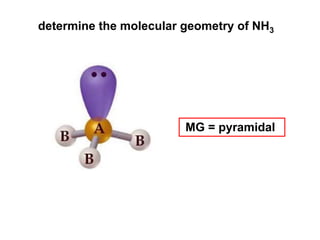
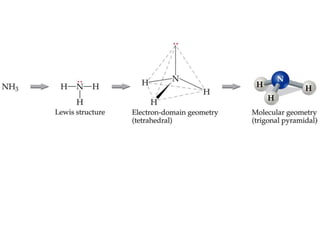
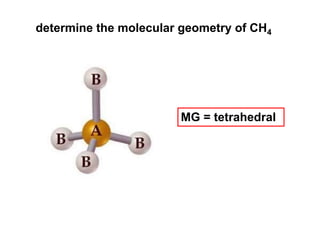
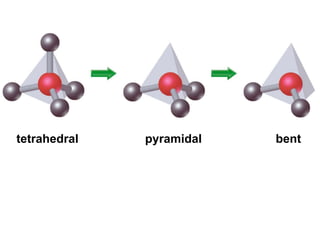
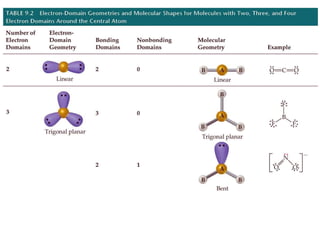
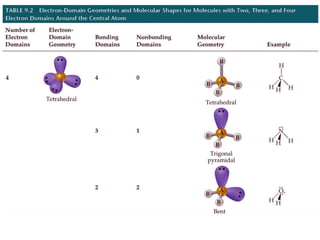
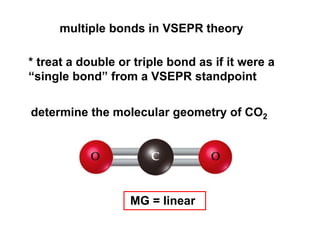
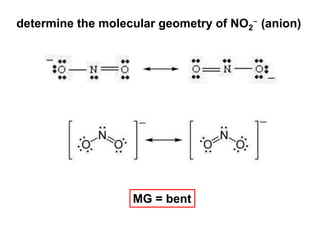
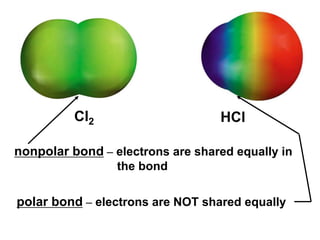
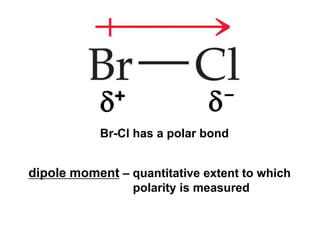
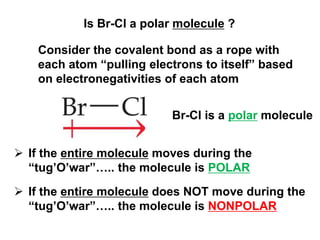
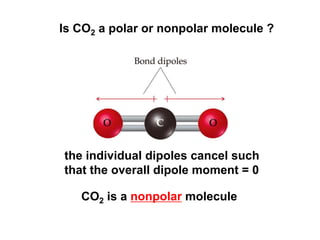
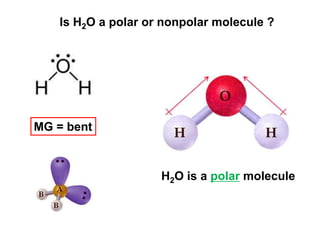
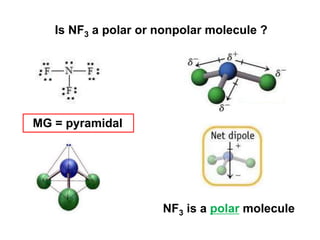
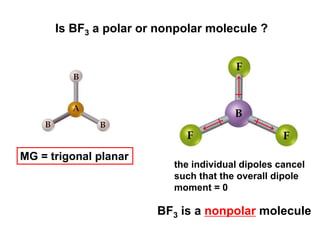
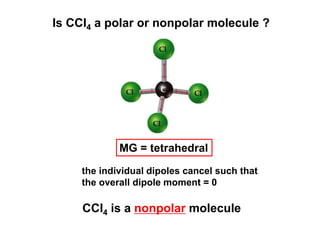
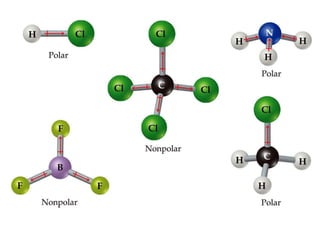
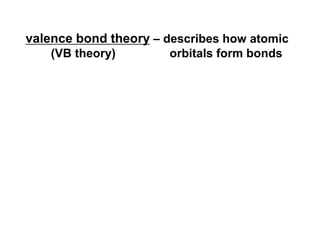
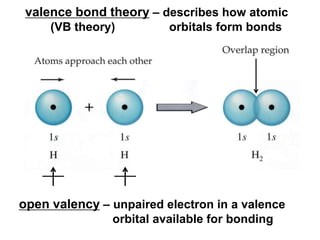
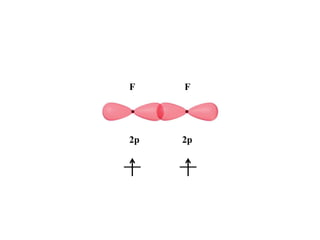
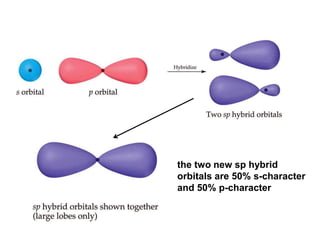
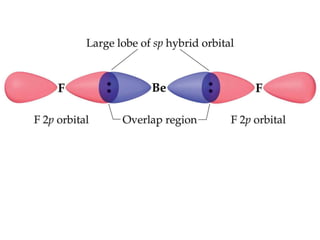
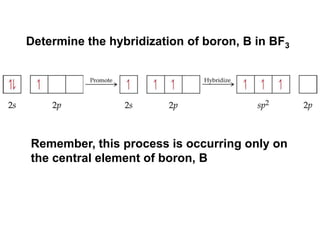
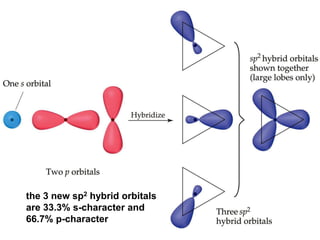
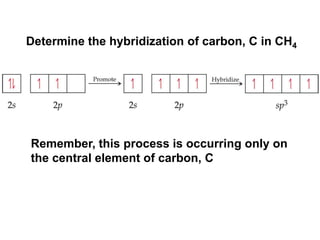
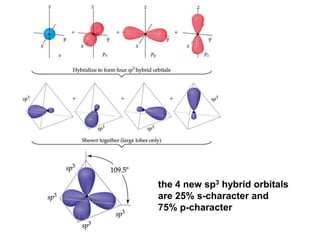
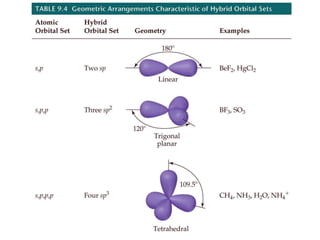
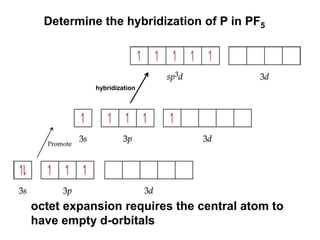
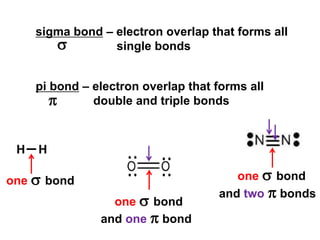
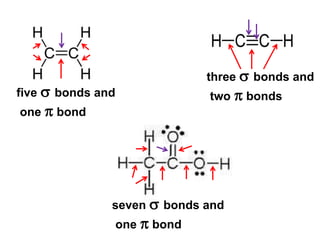
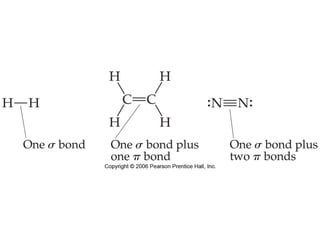
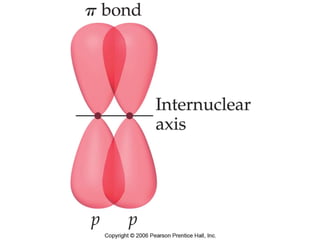
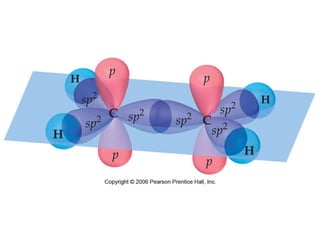
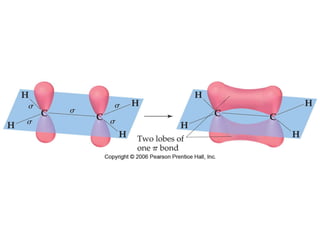
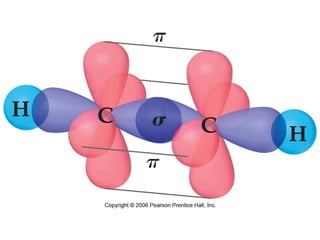
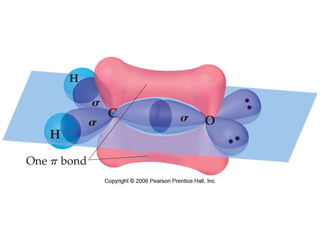
VSEPR theory predicts molecular geometry based on electron pair repulsion around a central atom. The number of electron pairs determines the molecular geometry, such as trigonal planar for 3 pairs or tetrahedral for 4 pairs. To determine molecular geometry, one draws the Lewis structure, counts electron pairs, and assigns an orientation to the atoms based on electron pair repulsion.