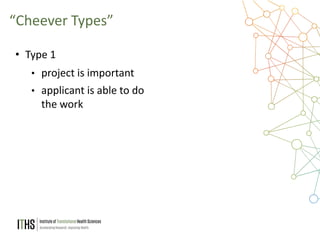
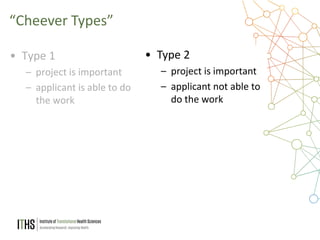
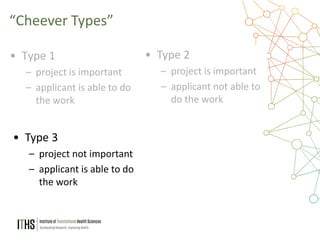
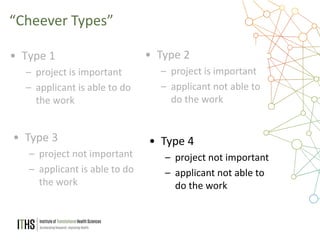
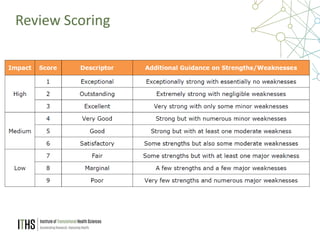
The document outlines the NIH study review process, led by Dr. Paul J. Martin, detailing key components such as meeting structure, scoring criteria, and recent changes emphasizing 'impact.' It provides guidance on writing applications, common mistakes, and factors affecting the review process while encouraging applicants to present unique and significant research contributions. Additional resources for further information are also included.