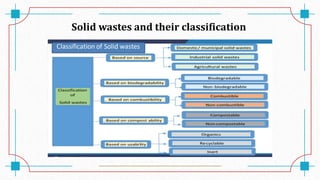
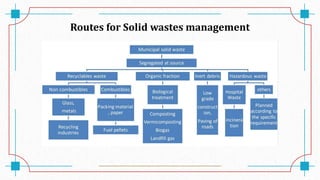
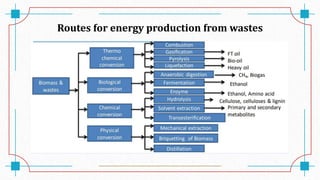
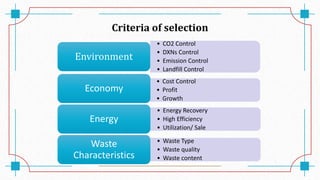
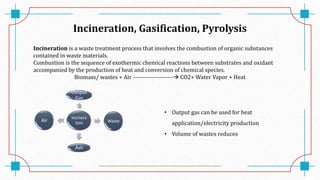
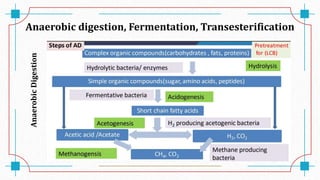
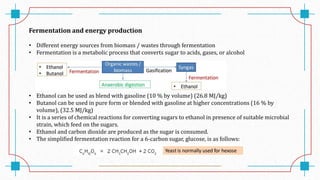
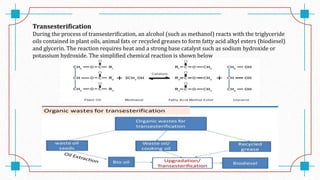
The document discusses waste-to-energy conversion. It introduces waste-to-energy as the process of generating energy from waste through combustion or production of fuels. The need for waste-to-energy is due to limited natural resources and increasing waste amounts. Methods discussed include incineration, gasification, pyrolysis, anaerobic digestion, and transesterification. Challenges include high capital costs, environmental skepticism, and lack of clear standards. The conclusion recommends an integrated solid waste management approach with public-private partnerships to address these challenges.