Recommended
PPT
Avogadros law and Ideal Gas Law.ppt
PPTX
GASES AND THERMO SCI DAMA.pptx111111111111
PDF
Complete Gas Laws Reviewer: Boyle’s, Charles’, Gay-Lussac, Dalton’s, Ideal Ga...
PPTX
Chem unit 12 presentation
PPTX
Ideal Gas Law and its calculations and behavior
PPTX
Chapter-5 Gas (Quiz-5).jwbfwfiuwehfioujoij23oipptx
PPTX
PPT
Cmcchapter13 100613153048-phpapp02
PPT
PPT
PPTX
PPTX
harmfulljunkdddddddddddddddddddddddddddddddddddddddddddddd
PDF
PPT
PRINCIPLES OF CHEMISTRY GASES
PPT
Ch5z5egases 110115225412-phpapp01
PDF
PPTX
Gas-Laws.pptx gas charles boyle gay lussac
PPT
Gases Chapter 13 gas laws boyles charless gay-lussacs
PPTX
PPTX
PPTX
Ideal Gas Equation and gas laws explaining the formulas and numericals
PPTX
PPT
PPT
D0596504 chem12 c14_l3_lo_mig
PPT
PDF
GAS LAWS PRESENTATION PROPER AND LESSONS
PPT
PPTX
Chemunit12presentation 120409192209-phpapp02
PPT
Gay-Lussac_s Gas Law.ppttttttttttttttttt
PPT
ChemReactions.pptssssssssssssssssssssssssssssssssssssssssssssssssssssssssssss...
More Related Content
PPT
Avogadros law and Ideal Gas Law.ppt
PPTX
GASES AND THERMO SCI DAMA.pptx111111111111
PDF
Complete Gas Laws Reviewer: Boyle’s, Charles’, Gay-Lussac, Dalton’s, Ideal Ga...
PPTX
Chem unit 12 presentation
PPTX
Ideal Gas Law and its calculations and behavior
PPTX
Chapter-5 Gas (Quiz-5).jwbfwfiuwehfioujoij23oipptx
PPTX
PPT
Cmcchapter13 100613153048-phpapp02
Similar to VISUAL ideal gas law.pptxxxxxxxxxxxxxxxxxx
PPT
PPT
PPTX
PPTX
harmfulljunkdddddddddddddddddddddddddddddddddddddddddddddd
PDF
PPT
PRINCIPLES OF CHEMISTRY GASES
PPT
Ch5z5egases 110115225412-phpapp01
PDF
PPTX
Gas-Laws.pptx gas charles boyle gay lussac
PPT
Gases Chapter 13 gas laws boyles charless gay-lussacs
PPTX
PPTX
PPTX
Ideal Gas Equation and gas laws explaining the formulas and numericals
PPTX
PPT
PPT
D0596504 chem12 c14_l3_lo_mig
PPT
PDF
GAS LAWS PRESENTATION PROPER AND LESSONS
PPT
PPTX
Chemunit12presentation 120409192209-phpapp02
More from ClaireMangundayao1
PPT
Gay-Lussac_s Gas Law.ppttttttttttttttttt
PPT
ChemReactions.pptssssssssssssssssssssssssssssssssssssssssssssssssssssssssssss...
PPTX
CHM2 11_12 Q3 0101 PF FD.pptxxxxxxxxxxxx
PPT
Naming and Writing Ionic-Covalent Compounds.ppt
PPTX
5partsofresearchpaper-130125220422-phpapp01.pptx
PPTX
FINAL-PS_PR-2-11_12_UNIT-8_LESSON-2_Recommendations-for-Quantitative-Research...
PPTX
FINAL-PS_PR-2-11_12_UNIT-6_LESSON-1_Practices-in-Collecting-Quantitative-Data...
PPTX
FINAL-PS_PR2-11_12_UNIT-7_LESSON-1_Descriptive-Statistics-for-Quantitative-Da...
PPTX
FINAL-PS_PR-2-11_12_UNIT-6_LESSON-3_Tabular-and-Graphical-Presentation-of-Qua...
PPTX
FINAL (PS)_PR 2 11_12_UNIT 6_LESSON 2_Summarizing Quantitative Data.pptx
PPTX
FINAL (PS)_PR2 11_12_UNIT 5_LESSON 1_Quantitative Research Design.pptx
PPTX
fosiiiiiiiiiiiillsssssssssssssssssssssss
PPTX
Science Biologyyyyyyyyyyyyyyyyyyyyyyyyyy
PPTX
Cells Structure and Function life.ppt.pptx
PPTX
fosiiiiiiiiiiiillsssssssssssssssssssssss
PPTX
fosiiiiiiiiiiiillsssssssssssssssssssssss
PPTX
Science Biologyyyyyyyyyyyyyyyyyyyyyyyyyy
PPTX
02 Cellsssssssssssssssssssssssssssssssss
PPTX
Science Biologyyyyyyyyyyyyyyyyyyyyyyyyyy
PPTX
Cell Types and Its function , kingdom of life
Recently uploaded
PDF
Basics of Systematic Literature Search - Nasra Gathoni
PDF
Nutrients & Role in Horticulture.pdf, Dr. Sharad Bisen Horticulture
PPTX
Cost of Capital - Cost of Equity, Cost of debenture, Cost of Preference share...
PDF
The Tale of Melon City poem ppt by Sahasra
PDF
The Tale of Melon City poem ppt by Sahasra
PDF
Principles and Practices of GST 2.0 Study material
PPTX
CLASS -9 POLITICAL SCIENCE PPT CHAPTER -5 DEMOCRATIC RIGHTS.pptx
PDF
1ST APPLICATION FOR ANNULMENT (4)8787666.pdf
DOCX
Mobile applications Devlopment ReTest year 2025-2026
PDF
NAVIGATE PHARMACY CAREER OPPORTUNITIES.pdf
PPTX
How to Automate Quality Checks in Odoo 18 Quality App
PDF
Blue / Green: Troop Leading Procedure (TLP) Overview.pdf
PPTX
Searching in PubMed andCochrane_Practical Presentation.pptx
PPTX
Details of Muscular-and-Nervous-Tissues.pptx
PPTX
Campfens "The Data Qualify Challenge: Publishers, institutions, and funders r...
PPTX
Rectal Surgery in Senior Citiizens .pptx
PDF
Projecte de la porta d'i5B: Els animals marins
PPTX
Pain. definition, causes, factor influencing pain & pain assessment.pptx
PDF
Projecte de la porta de la classe de primer A: Mar i cel.
PPTX
Unit I — Introduction to Anatomical Terms and Organization of the Human Body
VISUAL ideal gas law.pptxxxxxxxxxxxxxxxxxx 1. IDEAL GAS LAW
The ideal gas law, also called
the general gas equation, is the
equation of state of a
hypothetical ideal gas. It is a
good approximation of the
behavior of many gases under
many conditions, although it has
several limitations.
2. The Ideal Gas Equation is
useful in illustrating the
relationship among the
pressure, volume,
temperature, and number of
moles of a gas. This
equation is used to describe
gases that behave ideally.
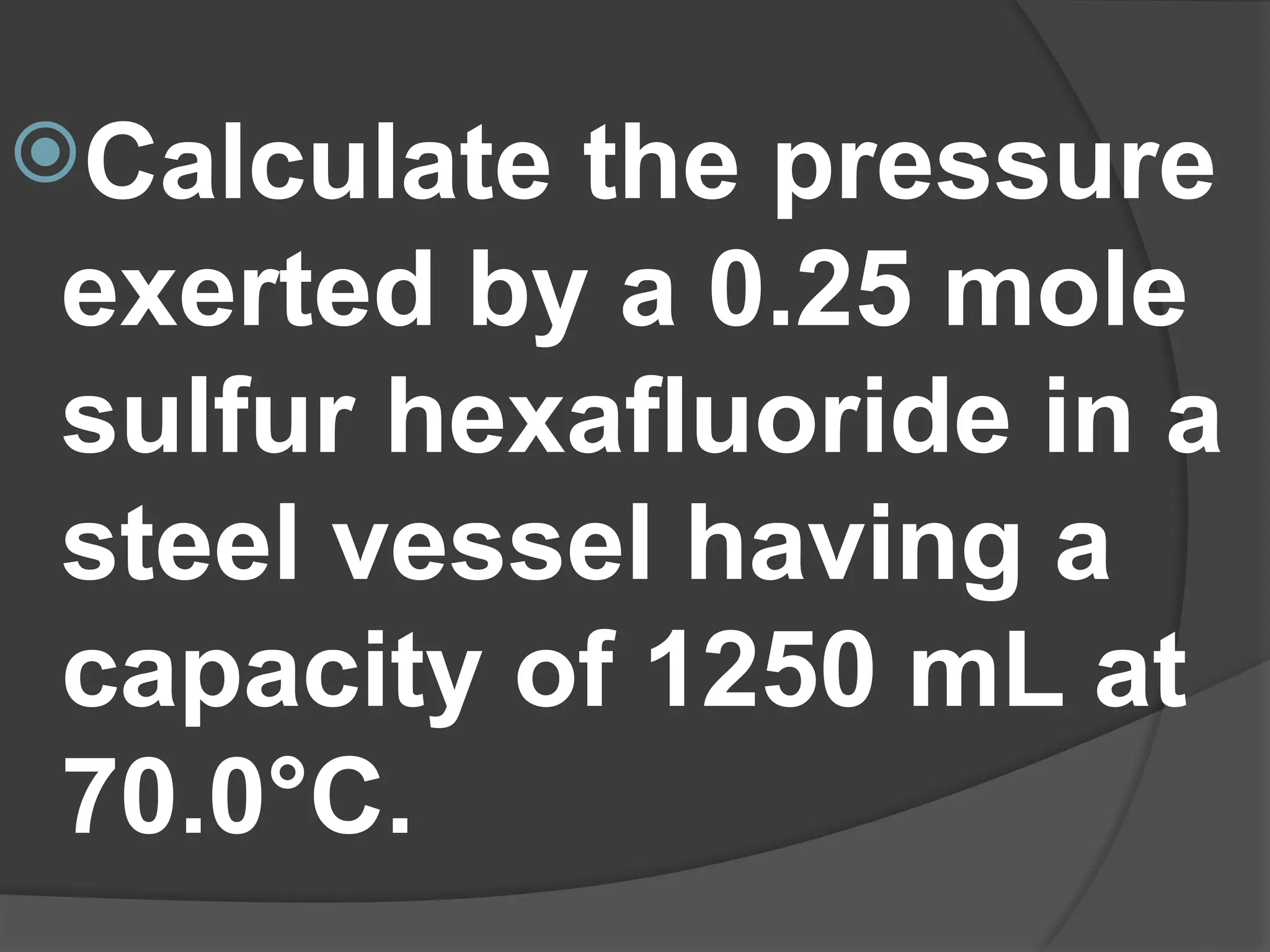
3. PV=nRT
where:
V = volume in liters
P = pressure in atmosphere
n = moles
T = temperature in Kelvin
R = universal gas constant,
0.0821 L. atm
4. What is the volume
of a container that
can hold 0.50 mole
of gas at 25.0°C and
1.25atm?
5. If I have an unknown
quantity of gas at a
pressure of 912 mm Hg,
a volume of 31,000 ml,
and a temperature of
87 0
C, how many moles
of gas do I have?
6. 7. A cylinder of Argon
gas contains 50.0 L of
Argon at 18.4 atm and
127 °C. How many
moles of argon is in
the cylinder?
8. Fermentation of glucose
produce gas in the form of
carbon dioxide, how many
moles of carbon dioxide is
produced if 0.78 L of carbon
dioxide at 20.1°C and 1.00
atm was collected during
the process?