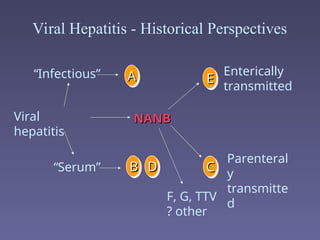
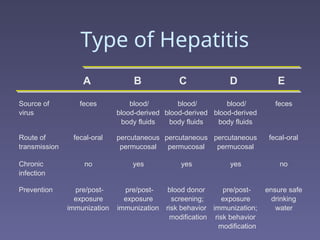
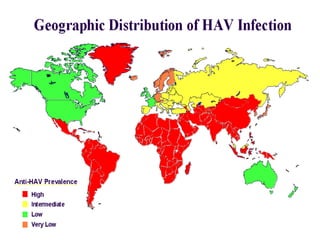
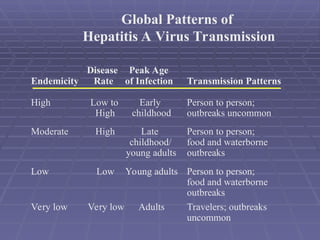
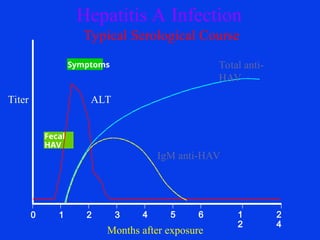
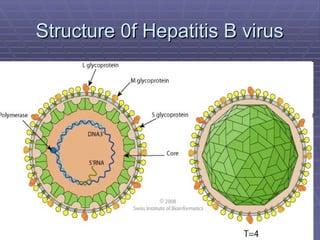
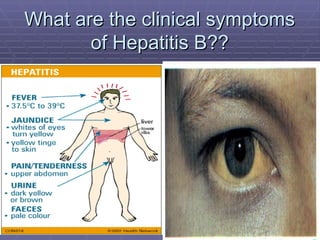
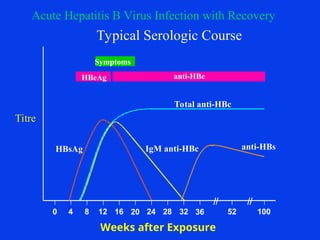
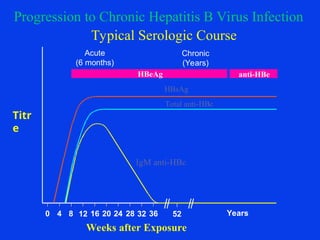
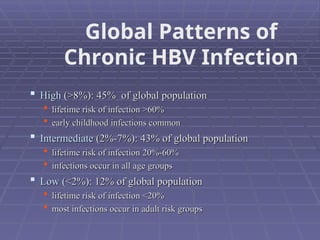
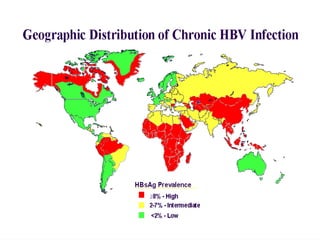
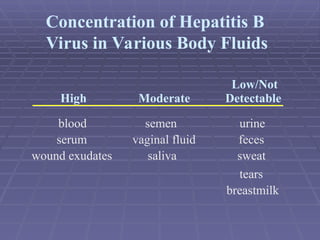
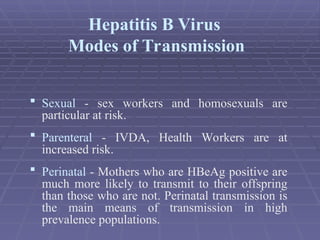
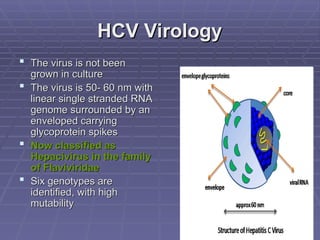
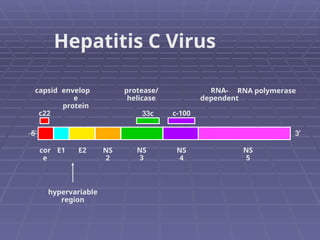
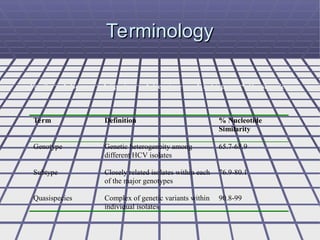
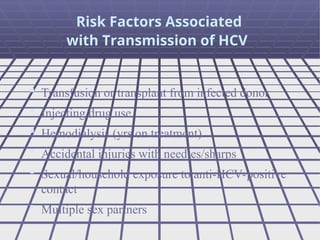
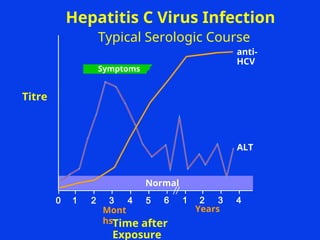
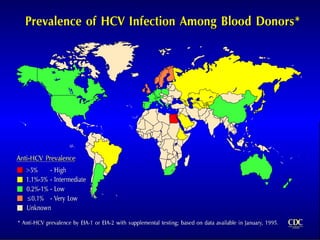
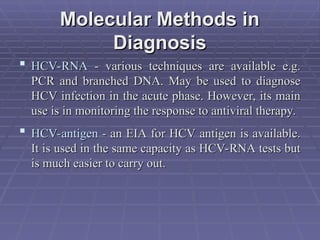
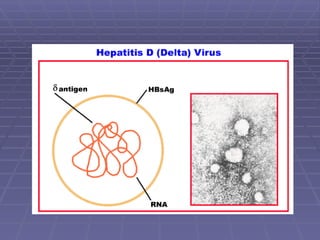
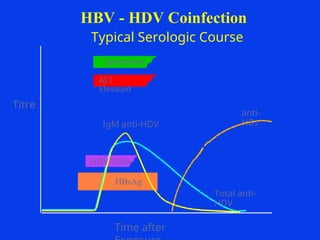
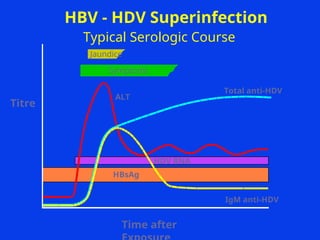
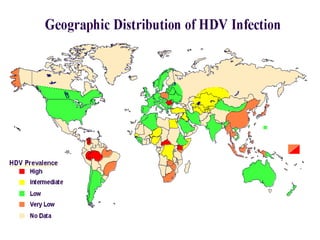
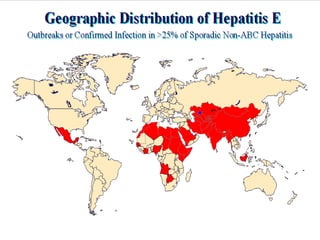
Hepatitis refers to inflammation of the liver caused by various factors, primarily viral infections such as hepatitis A, B, and C. The document explains the nature, transmission, clinical manifestations, prevention, and vaccination strategies for these viral hepatitis types, emphasizing the importance of sanitation and vaccination in reducing disease incidence. It highlights the difference between acute and chronic cases, the epidemiology of the infections, and the necessity of public health measures to control outbreaks.