This document provides an introduction to vestibular evoked myogenic potentials (VEMP). It discusses how VEMP responses in muscles like the sternocleidomastoid muscle can be elicited by sound, vibration, or electrical stimulation and assessed clinically. While early studies recorded potentials from the inion, the current clinical form of VEMP testing utilizes biphasic responses recorded from the sternocleidomastoid muscle. VEMP testing was established as a method to assess saccular function after studies found the responses disappeared on the affected side after unilateral vestibular nerve section. Further research has since clarified that sound stimulation of the saccule is conveyed by the inferior vestibular nerve, making VEMP a useful








![Introduction
Vestibular evoked myogenic potentials (VEMP) are responses in the muscles,
especially cervical muscles such as the sternocleidomastoid muscle (SCM), to
sound, vibration, or electrical stimulation (Fig. 1). Because it seemed that VEMP
could be used for clinical tests of the vestibular end-organs, especially the saccule,
it attracted the interest of clinicians and scientists. There had been no other clinical
test of the saccule that was applicable at common clinics. Now much has been
published about VEMP, and many clinicians use this test. VEMP is one of the most
important advances in clinical neurophysiology of the vestibular system.
Prior to the availability of VEMP in its present form [1], other tests had been
proposed, such as using inion responses [2, 3] (Fig. 2), which tried to measure
potentials evoked by sound as a test of the vestibular system. However, tests relying
on these responses were not widely used at clinics. VEMP in its present form,
utilizing biphasic myogenic potentials on the SCM, were first reported in 1992 by
Colebatch and Halmagyi, who reported that VEMP responses on the affected side
disappeared after unilateral vestibular nerve section despite preservation of hearing
[1, 4] (Fig. 3). Colebatch et al. reported in 1994 that VEMP could be recorded in
a patient with bilateral near-total hearing loss [4]. Since that report, clinical and
basic studies concerning VEMP have been further developed. These later studies
clarified that the major vestibular end-organ which responds to sound is the saccule,
and that signals are conveyed via the inferior vestibular nerve [5–11]. Details are
given in the chapters that follow. Here, we want to emphasize that the progress
achieved in this field was brought about by the collaboration of scientists and
clinicians.
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 3
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_1, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-9-320.jpg)
![4 Vestibular Evoked Myogenic Potentials
Fig. 2. A typical waveform of inion responses. An active electrode was placed on the nasion and
a reference electrode was on the mastoid. 70dBnHL clicks were presented binaurally; 150
responses were averaged. (From [3], with permission)
10 msec
100 mV
p13
n23
n34
p44
䉱
click
Fig. 1. A typical waveform of vestibular evoked myogenic potentials (VEMP) in a healthy
subject](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-10-320.jpg)
![Introduction 5
Fig. 3. Abolishment of VEMP responses (to 100-dB clicks) following selective vestibular nerve
section on the left. VEMP responses on the left sternocleidomastoid muscle (SCM) to left ear
stimulation were absent after unilateral vestibular nerve section, although hearing on the left was
preserved. Upper left, recording on the right SCM to left ear stimulation; upper right, recording
on the right SCM to right ear stimulation (presence of responses); asterisk, p13; lower left, record-
ing on the left SCM to left ear stimulation (absence of responses); lower right, recording on the
left SCM to right ear stimulation. (From [4], with permission)
References
1. Colebatch JG, Halmagyi GM (1992) Vestibular evoked potentials in human neck muscles
before and after unilateral vestibular deafferentation. Neurology 42:1635–1636
2. Bickford RG, Jacobson JL, Cody DT (1964) Nature of average evoked potentials to sound
and other stimuli in man. Ann NY Acad Sci 112:204–223
3. Cody DT, Jacobson JL, Walker JC, et al (1964) Averaged evoked myogenic and cortical
potentials to sound in man. Ann Otol Rhinol Laryngol 73:763–777
4. Colebatch JG, Halmagyi GM, Skuse NF (1994) Myogenic potentials generated by a
click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 57:190–197
5. McCue MP, Guinan JJ Jr (1995) Spontaneous activity and frequency selectivity of
acoustically responsive vestibular afferents in the cat. J Neurophysiol 74:1563–1572
6. McCue MP, Guinan JJ Jr (1997) Sound-evoked activity in primary afferent neurons of a
mammalian vestibular system. Am J Otol 18:355–360
7. Murofushi T, Curthoys IS, Topple AN, et al (1995) Responses of guinea pig primary vestibu-
lar neurons to clicks. Exp Brain Res 103:174–178
8. Murofushi T, Curthoys IS, Gilchrist DP (1996) Response of guinea pig vestibular nucleus
neurons to clicks. Exp Brain Res 111:149–152
9. Murofushi T, Curthoys IS (1997) Physiological and anatomical study of click-sensitive
primary vestibular afferents in the guinea pig. Acta Otolaryngol (Stockh) 117:66–72
10. Murofushi T, Halmagyi GM, Yavor RA, et al (1996) Absent vestibular evoked potentials in
vestibular neurolabyrinthitis: an indicator of involvement of the inferior vestibular nerve?
Arch Otolaryngol Head Neck Surg 122:845–848
11. Murofushi T, Matsuzaki M, Mizuno M (1998) Vestibular evoked myogenic potentials in
patients with acoustic neuromas. Arch Otolaryngol Head Neck Surg 124:509–512](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-11-320.jpg)

![Overview of the Vestibular System
Introduction
In this chapter, we review only the fundamental structures associated with the
vestibular system that may be concerned with vestibular evoked myogenic
potentials (VEMPs). Although the cerebellum and cerebrum are also important
for the vestibular system, we do not address them here, as their effects on VEMPs
seem minimal.
Vestibular End-Organs
The human labyrinth consists of the cochlea, otolith organs, and semicircular
canals. The otolith organs and the semicircular canals are vestibular end-organs.
The functions of the vestibular end-organs are basically to monitor the rotational
and linear movement of the head and the orientation of the head to gravity. In
humans, there are two otolith organs (saccule and utricle) and three semicircular
canals (lateral semicircular canal, anterior semicircular canal, and posterior semi-
circular canal) (Figs. 1, 2).
The otolith organs, the saccule and the utricle, sense linear acceleration. The
sensory area of the otolith organ is called the macula. The saccular macula lies on
the medial wall of the vestibule in a spherical recess inferior to the utricular macula.
The saccular macula is hook-shaped and lies predominantly in a vertical position,
whereas the utricular macula is oval and lies predominantly in a horizontal position
(Fig. 3) [1] The plane of the saccular macula is almost orthogonal to that of the
utricular macula (Fig. 4). The surfaces of the maculae are covered by the otolithic
membrane, which contains a superficial calcareous deposit, the otoconia (Fig. 5)
[1–3]. The cilia of the hair cells in the macula protrude into the otolithic membrane.
The otoconia consist of small calcium carbonate crystals [4]. Linear acceleration
including gravity causes deflection of the cilia of the hair cells.
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 9
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_2, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-13-320.jpg)
![10 Vestibular Evoked Myogenic Potentials
The semicircular canals—lateral, anterior, posterior—sense angular accelera-
tion. They are aligned to form a coordinate system [5]. The lateral semicircular
canal makes a 30° angle with the horizontal plane. The other two canals are in
vertical positions almost orthogonal to each other. The sensory area is called the
crista. Hair cells are in the surface of the crista, with their cilia protruding into
the cupula, a gelatinous mass (Fig. 6). Angular acceleration causes deflection of
the cupula, resulting in deflection of the cilia of the hair cells.
Utricle
Saccule
Anterior semicircular canal
Posterior semicircular canal
Lateral semicircular canal Cochlea
Cochlear nerve
Superior vestibular nerve
Inferior vestibular nerve
(a) (b)
(c)
Fig. 1. Inner ear and afferent nerves
Fig. 2. Vestibular end-organs in human temporal bone sections. a Utricular macula. b Saccular
macula. c Crista of the lateral semicircular canal](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-14-320.jpg)

![12 Vestibular Evoked Myogenic Potentials
The macula and crista contain two types of hair cells: type I and type II hair
cells [6] (Fig. 7). The hair cells have stereocilia and a kinocilium on the top. Deflec-
tion of the stereocilia toward the kinocilium causes excitation, whereas deflection
toward the other side causes inhibition. Type I hair cells are shaped like flasks, with
each cell being surrounded by a calyx ending. The type II hair cells, which are like
cylinders, have bouton nerve endings (Figs. 7, 8) [1, 2]. The striola is a distinctive
cupula
hair cell
Fig. 6. Photomicroscopic findings of the guinea pig crista. H&E
kinocilium
stereocilia
excitation
inhibition
calyx type ending
bouton type ending
Type I
Type II
Fig. 7. Two types of hair cell](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-16-320.jpg)
![Overview of the Vestibular System 13
zone running through the center of each macula. The hair cells on each side of the
striola have opposite polarities because their kinocilia point in opposite directions
(Fig. 3). In contrast to the macula, the direction of the polarity of hair cells in one
crista is uniform.
Calyx units are seen in central (striolar) zones. The axon is usually unbranched,
giving rise to a single calyx ending. Bouton units are seen in peripheral (extrastrio-
lar) zones. The axon provides bouton endings to several type II hair cells. Dimor-
phic units innervate all parts of the neuroepithelium. The axon has collateral
branches terminating as calyx endings and bouton endings [6].
Vestibular Nerve
The vestibular nerve contains afferents from the vestibular end-organs and effer-
ents. The cells of afferents are bipolar neurons with their cell bodies in Scarpa’s
ganglion. The vestibular nerve is subdivided into two parts: the superior vestibular
nerve and the inferior vestibular nerve [7] (Fig. 1). The superior vestibular nerve
innervates the cristae of the anterior semicircular canal, the lateral semicircular
canal, the utricular macula, and the anterosuperior part of the saccular macula. The
inferior vestibular nerve innervates the crista of the posterior semicircular canal
and the main part of the saccular macula. Otolith ganglion cells are located ven-
trally in the central portion of the ganglion, whereas canal ganglion cells are located
at the rostral and caudal ends [8, 9].
The vestibular afferent fibers innervating the macula are activated by changes
in the position of the head in space or by linear acceleration, whereas the fibers
innervating the crista are activated by angular acceleration [10–12]. Vestibular
afferents fire spontaneously (65 spikes/s in otolith afferents and 90 spikes/s in canal
afferents) [13, 14]. The baseline firing rates increase in response to excitatory
stimuli and decrease in response to inhibitory stimuli. Based on the regularity of
firing, vestibular afferents are classified into two groups—regularly firing fibers
and irregularly firing fibers [6]—each of which has different features. Irregularly
Bouton type ending
Calyx type ending
Fig. 8. Types of guinea pig primary afferent nerve endings labeled by biocytin. (In collaboration
with Prof. I.S. Curthoys)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-17-320.jpg)
![14 Vestibular Evoked Myogenic Potentials
firing fibers have thick, medium-sized axons ending as a calyx and dimorphic units.
They have phasic–tonic response dynamics and high sensitivity to head rotation or
linear forces. In contrast, regularly firing fibers have medium-sized, thin axons,
ending as dimorphic and bouton units. They have tonic response dynamics and low
sensitivity to head rotation or linear forces. These differences must be borne in
mind when we consider the sound sensitivity of vestibular afferents.
Vestibular Nucleus
The vestibular nuclei consist of a group of neurons located on the floor of the
fourth ventricle. The main vestibular nuclei are the superior nucleus, lateral
(Deiters’) nucleus, medial nucleus, and inferior (descending or spinal) nucleus (2).
Additionally, there are several small groups of cells. Although primary vestibular
neurons provide multiple branches, which usually innervate secondary vestibular
neurons in all of the four main vestibular nuclei, there are preferences in each
nucleus (8).
The superior vestibular nucleus contains medium-sized neurons with some mul-
tipolar cells. The superior vestibular nucleus receives strong projections from
semicircular canals. The medial vestibular nucleus consists of cells of many sizes
and shapes that are close together. The upper part of the medial vestibular nucleus
receives fibers from the semicircular canals and the fastigial nucleus and flocculus
of the cerebellum. Saccular and utricular afferents project to the middle part of
the nucleus. The caudal part of the nucleus receives fibers from the cerebellum.
The lateral vestibular nucleus contains giant cells. The dorsocaudal portion
receives afferents from the cerebellum, whereas the rostrovertebral portion receives
primary vestibular afferents. The inferior vestibular nucleus consists of small and
medium-sized cells with occasional giant cells. The rostral part of the inferior
vestibular nucleus receives strong projections from the otolith organs and the
semicircular canals.
Summarized from the standpoint of primary otolith afferents, saccular afferents
terminate mainly in the rostral part of the inferior vestibular nucleus and the ros-
troventral portion of the lateral nucleus; and utricular afferents terminate mainly in
the rostral portion of the inferior vestibular nucleus, and the medial vestibular
nucleus [8, 9, 15].
On the basis of neurophysiological studies, two types of secondary vestibular
neuron were identified [16]. Ipsilateral rotation of the head causes type I neurons
to be excited and type II neurons to be inhibited. Type I neurons are monosy-
naptically activated by ipsilateral primary afferents, whereas type II neurons
receive inputs via commissural connections either from neurons in the reticular
substance or directly from contralateral type I neurons [2]. Contralateral labyrinth
stimulation excites type II neurons, resulting in inhibition of type I neurons
(Fig. 9).](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-18-320.jpg)
![Overview of the Vestibular System 15
Vestibulospinal Reflex
The vestibulospinal reflex (VSR) serves to stabilize the head and controls erect
stance relative to gravity under both static and dynamic conditions [17]. Stimula-
tion of the vestibular end-organs leads to various patterns of activation of neck
and body muscles. Activation of the neck muscles is described later as the vestibu-
locollic reflex.
The VSR prevents falling and maintains the body’s position. There are three
major pathways: the lateral vestibulospinal tract (LVST); the medial vestibulospinal
tract (MVST); and the reticulospinal tract (RST) [18] (Fig. 10). The LVST origi-
nates in the lateral nucleus and descends in the ipsilateral ventral funiculus of the
spinal cord. The MVST originates in the medial, inferior, and lateral nuclei and
descends in the medial longitudinal fasciculus bilaterally as far as the mid-thoracic
level [17, 19]. Linear and angular head acceleration causes increased muscle tones
in the ipsilateral extensor muscles and decreased muscle tones in the ipsilateral
flexor muscles via the LVST [17, 20].
Vestibulocollic Reflex
The vestibulocollic reflex (VCR) operates to stabilize the head in space by neck
movements. The MVST and LVST provide direct connections to neck motoneurons
as well as indirect connections. Connections between vestibular end-organs and
Fig. 9. Interrelation of types I and II secondary vestibular neurons. Light neurons are excitatory;
dark neurons are inhibitory
Type I
Type II
Type II
Type I
Lateral
semiciicular canal
Vestibular nerve
Vestibular nucleus
midline](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-19-320.jpg)

![Overview of the Vestibular System 17
neck motoneurons are summarized in Table 1 [21]. Neck muscles are classified
into three types: extensors, flexors, rotators. The sternocleidomastoid muscle (SCM)
is classified as a rotator type. Concerning VEMPs, it should be noted that moto-
neurons of the SCM have disynaptic inhibitory inputs from the ipsilateral saccule
with no projections from the contralateral saccule [22] (Table 1, Fig. 11).
Vestibuloocular Reflex
The vestibuloocular reflex (VOR) maintains gaze during head and body movements
[23]. This gaze stability is achieved by activation of vestibular end-organs, includ-
ing activation of semicircular canals to angular acceleration and of otolith organs
to linear translation and gravity. In other words, the VOR produces extraocular
muscle contraction to compensate for a specific head movement, thereby maintain-
ing gaze stability. The connections of the semicircular canals with extraocular
muscles are summarized in Table 2.
The eye movements induced by the stimulation of the otolith organs and the
pathways from the otolith organs to extraocular muscles are less clearly defined
than those from the semicircular canals and are somewhat controversial. According
to Suzuki et al. [24], electrical stimulation of the utricular nerve in spinalized, alert
cats mainly produced contraversive torsional eye movement with simultaneous
upward shift in ipsilateral eyes, downward shift in contralateral eyes, and slight
Fig. 11. Sacculosternocleidomastoid (SCM) and utriculosternocleidomastoid pathways Filled
circles, inhibitory neurons; open circles, excitatory neurons. (from Fig. 4 of ref. 22, Springer, with
permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-21-320.jpg)
![18 Vestibular Evoked Myogenic Potentials
contralateral horizontal shift of both eyes. Curthoys reported that electrical stimula-
tion of the utricular macula in guinea pigs produced upward or upward-torsional
eye movements [25]. Fluur and Mellstrom reported that electrical stimulation of
the utricular macula in alert cats produced eye movements whose direction depended
on the location of the stimulating electrode [26]. Concerning horizontal eye
movement, Goto et al. [27] confirmed that utricular nerve stimulation in cats
evoked horizontal eye movements to the stimulated side, supporting prior findings
of projection of the utricular nerve to the ipsilateral abducens nucleus [28, 29].
Eye movement due to saccular stimulation is more obscure than that due to
utricular stimulation. The saccule contributes more weakly to eye movements than
the semicircular canals or the utricule [30]. Previous studies suggested that the main
eye movement induced by saccular stimulation could be vertical [25, 31, 32].
References
1. Schuknecht HF (1993) Pathology of the ear. 2nd edn. Lea & Febiger, Philadelphia
2. Baloh RW, Honrubia V (1990) Clinical neurophysiology of the vestibular system. 2nd edn.
Davis, Philadelphia
3. Lim DJ (1973) Ultrastructure of the otolithic membrane and the cupula. Adv Otorhinolaryn-
gol 19:35–49
4. De Vries H (1951) The mechanics of the labyrinth otoliths. Acta Otolaryngol 38:262–273
5. Blanks RHJ, Curthoys IS, Markham CH (1975) Planar relationships of the semicircular canals
in man. Acta Otolaryngol 80:185–196
6. Goldberg JF, Lysakowski A, Fernandez C (1992) Structure and function of vestibular nerve
fibers in the chinchilla and squirrel monkey. Ann NY Acad Sci 656:92–107
7. Lorente De No R (1933) Anatomy of the eighth nerve: the central projection of the nerve
endings of the internal ear. Laryngoscope 43:1–38
8. Gacek RR (1969) The course and central termination of first order neurons supplying
vestibular end organs in the cat. Acta Otolaryngol 254:1–66
9. Gacek RR (2008) A place principle for vertigo. Auris Nasus Larynx 35:1–10
10. Fernandez C, Goldberg JM (1971) Physiology of peripheral neurons innervating semicircular
canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of periph-
eral vestibular system. J Neurophysiol 34:61–675
Table 2. Connection pattern to motoneurons of extra-ocular
muscles from afferents of the semicircular canals
Semicircular canal Excitation Inhibition
Anterior I-SR I-IR
C-IO C-SO
Posterior I-SO I-IO
C-IR C-SR
Lateral I-MR C-MR
C-LR I-LR
(from ref. 21)
I, ipsilateral; C, contralateral; MR, mdial rectus;
LR, lateral rectus; SO, superior oblique; IR, inferior rectus;
IO, inferior oblique; SR, superior rectus](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-22-320.jpg)

![Sound Sensitivity of the Vestibular
End-Organs and Sound-Evoked
Vestibulocollic Reflexes in Mammals
Sound Sensitivity of the Vestibular System
Tullio first reported that surgical fenestration of the bony labyrinth in avians and
mammals made the labyrinth sound-sensitive [1–3]. This phenomenon—sound
sensitivity of the vestibular system—has been known as the Tullio phenomenon
[3, 4]. Bekesy reported head movements in response to relatively loud sounds [3, 5]
and suggested that this effect might be caused by stimulation of the otolith organs.
Later, Young et al. reported that primary vestibular afferents of squirrel monkeys
could respond to sound and vibration, although the number of examined vestibular
neurons was limited and the methods of threshold determination (a phase-locking
threshold) were not familiar [6]. According to their study, all the vestibular end-
organs (three canals and two maculae) responded to sound. Among the five end-
organs, the saccular macula showed the lowest thresholds. The best frequencies did
not exceed 1000 Hz to sound and 500 Hz to vibration. Cazals et al. created guinea
pigs with selective cochlear loss and preserved the vestibular system using amika-
sin, an aminoglycoside. These animals displayed evoked potentials to sound,
although their cochlea was completely destroyed [7, 8]. Recording evoked poten-
tials on the eighth nerve revealed that the responses were prominent on the inferior
vestibular nerve [9]. These studies suggested that the vestibular end-organs could
respond to loud sounds and that the saccule might be the most sound-sensitive
among the vestibular end-organs.
During the 1990s, several articles concerning sound sensitivity of vestibular
neurons were published. McCue and Guinan reported that saccular afferents of cats
responded to intense sound. In their study, the best frequency of saccular afferents
to air-conducted sound was around 500 Hz (Fig. 1) [10–12]. Murofushi et al.
showed that primary vestibular afferents of guinea pigs could respond to intense
air-conducted clicks (Fig. 2) [13, 14]. These neurons were mainly in the inferior
vestibular nerve and could also respond to static tilts. None of the angular accelera-
tion-sensitive neurons—canal neurons—responded to clicks. These findings sug-
gested that the saccular afferents could be sensitive to air-conducted sound. Most
of these click-sensitive neurons showed irregular spontaneous firing. Irregularly
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 20
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_3, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-24-320.jpg)
![Sound Sensitivity and Sound-Evoked Vestibulocollic Reflexes 21
firing fibers have thick, medium-sized axons ending as calyx and dimorphic units
[15]. Hence, type I hair cells on the saccular macula are sound (click)-sensitive
among the vestibular end-organs. Murofushi et al. also reported that vestibular
nucleus neurons in the lateral vestibular nucleus and in the rostral portion of the
inferior vestibular nucleus are sound (click)-sensitive (Fig. 3) [16]. Although these
findings imply that saccular afferents could respond to air-conducted sound, they
do not exclude the possibility that utricular afferents might respond to air-con-
ducted sound as well, especially to relatively low-frequency sound.
In the vestibular end-organs, response patterns to bone-conducted sound (vibra-
tion) are different from the patterns seen with air-conducted sound. Hair cells in
Fig. 1. Tuning curves of sound-sensitive vestibular afferents of cats. SPL, sound pressure level.
(from Fig. 6 of ref. 11, American Physiological Society, with permission)
Fig. 2. Responses of guinea pig primary vestibular neurons to clicks—70 dB above the auditory
brainstem response (ABR) threshold. (from Fig. 1 of ref. 14, Taylor & Francis, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-25-320.jpg)
![22 Vestibular Evoked Myogenic Potentials
the utricular macula and the saccular macula respond to bone-conducted sound.
According to Curthoys et al., most of the irregular otolithic afferents studied
(82.8%) showed a clear increase in the firing rate in response to bone-conducted
sound [17]. In their study, bone-conducted sound-sensitive afferents could be of
utricular origin because many of the bone-conducted sound-sensitive afferents were
in the superior vestibular nerve, and they were sensitive to roll tilts. These authors
also reported that regular otolithic afferents were less sensitive to bone-conducted
sound, and only a few canal afferents responded to it. These findings suggested
that vestibular evoked myogenic potentials (VEMPs) to bone-conducted sound
[18, 19] might be produced by vestibular end-organs from a different population
than the VEMPs to air-conducted sound.
Neural Pathway of the Sound-Evoked Vestibulocollic Reflex
Among the vestibular end-organs in mammals, the saccular macula seems to
respond especially well to air-conducted sound; and among the hair cells on the
saccular macula, the type I hair cells around the striola seem to be the most sensi-
tive. What then is the neural pathway of the sound-evoked vestibulocollic reflex?
Primary afferents of the saccule are mainly in the inferior vestibular nerve.
Therefore, inputs to the vestibular system of sound stimulation are mostly trans-
mitted via the inferior vestibular nerve. According to Kushiro et al. [20], saccular
Fig. 3. Recording sites of click-sensitive vestibular nucleus neurons of guinea pigs. LV, lateral
vestibular nucleus; DV, descending vestibular nucleus; MV, medial vestibular nucleus; SV, supe-
rior vestibular nucleus; icp, inferior cerebellar peduncle; CN, cochlear nucleus; as, acoustic stria;
n7, facial nerve; g7, genu nervi facialis; N6, abducens nucleus. a is the most rostral and c is the
most caudal. (from Fig. 2 of ref. 16, Springer, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-26-320.jpg)
![Sound Sensitivity and Sound-Evoked Vestibulocollic Reflexes 23
afferents in cats have inhibitory projection to the ipsilateral motoneurons of the
sternocleidomastoid muscle (SCM) and no contralateral projection. These authors
also showed that this projection was transmitted via the medial vestibulospinal
tract. Based on these findings, the neural pathway of the air-conducted sound-
evoked vestibulocollic reflex recorded on the SCM is thought to be as shown in
Fig. 4. The VEMPs are clearly ipsilateral-dominant (described later). Therefore,
the supposed neural pathway corresponds well with the results of VEMP studies
in humans.
Provided that bone-conducted sound simulates the utricular macula as well as
the saccular macula, VEMPs to bone-conducted sound might have some features
different from those of VEMPs to air-conducted sound. Utricular afferents are
located in the superior vestibular nerve. Utricular afferents have not only inhibitory
projection to the ipsilateral motoneurons of the SCM but also excitatory projection
to the contralateral motoneurons of the SCM [20]. When one uses bone-conducted
sound as the stimulus, one should bear in mind that the neural pathway of VEMPs
for bone-conducted sound might be different from the pathway of VEMPs for air-
conducted sound.
When sound is presented to the ear, one may concern the coexistence of a
“sound-evoked cochleocollic reflex”. However, direct projection from the cochlear
nucleus to the motoneurons of the SCM is not known. Therefore, the sound-evoked
cochleocollic reflex, if any, would be transmitted via the reticular formation, taking
longer latencies than the sound-evoked vestibulocollic reflex. Furthermore, it would
be evoked bilaterally. Some of the later components of VEMPs (n34–p44) [21]
may be a sound-evoked cochleocollic reflex.
Fig. 4. Pathway of air-conducted sound-evoked vestibulocollic (otolith-sternocleidomastoid)
reflex. SCM, sternocleidomastoid muscle
inferior vestibular nerve
medial vestibulospinal tract
accessory nerve
ipsilatral SCM
saccule](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-27-320.jpg)

![Recording and Assessing VEMPs
Suitable Subjects for Recording VEMPs
Basically, vestibular evoked myogenic potential (VEMP) testing is applicable to
all subjects who require evaluation of vestibular functions. However, it is difficult
to obtain responses from subjects who are not cooperative during the testing and
who for some reason cannot contract the sternocleidomastoid muscle (SCM) during
the recording (e.g., a comatose patient). In subjects with air–bone gaps in pure-tone
audiometry, special care is required because responses are abolished or decreased
owing to conductive hearing loss [1, 2].
Methods of Recording VEMPs
We usually use surface electrodes to record VEMPs, placing active electrodes sym-
metrically on the middle third of the SCM and indifferent electrodes on the lateral
end of the upper sternum (Fig. 1) [3]. When the active electrodes are too close to
the indifferent electrodes, the amplitudes of the responses are decreased [4]; and
when they are too close to the mastoid, responses are contaminated by postauricular
responses [5]. The ground electrode is placed on the nasion or the chin.
Acoustic stimuli usually comprise clicks (0.1 ms) or short tone bursts (STBs)
(500 Hz, rise/fall time 1 ms, plateau time 2 ms). We first present 95-dBnHL
(decibels, normal hearing level) clicks or STBs and attenuate the intensity when
we want to determine the threshold of the responses. STBs of 500 Hz evoke larger,
clearer VEMP responses than clicks [6]. However, investigators should note that
STBs of 500 Hz might evoke utricular hair cells as well as saccular hair cells,
whereas clicks selectively evoke saccular hair cells [7, 8]. The repetition rate of
stimulation is usually 5 Hz. When the repetition rate is increased, the amplitude of
the responses may be decreased. This tendency becomes clear when the repetition
rate is more than 20 Hz [9]. On the other hand, subjects may become tired when
the repetition rate is decreased because the lower repetition rate requires contraction
of the SCM for longer periods. Thus, 5 Hz is the optimal repetition rate.
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 25
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_4, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-29-320.jpg)
![26 Vestibular Evoked Myogenic Potentials
Electromyographic (EMG) activities are amplified and bandpass-filtered (20–
2000 Hz). The time window for analysis is 50–100 ms. Responses to 100–200
stimuli (click VEMPs) are averaged. It is important to maintain the contraction
of the SCM during recording. VEMP amplitudes show strong correlations to
background muscle activity (Fig. 2). Responses cannot be observed without muscle
contraction.
As VEMP amplitudes show strong correlations with the extent of muscle con-
traction, efforts to minimize the effects of muscle contraction fluctuation may be
required. To minimize such effects, it is proposed that 1) VEMP amplitudes be
corrected based on the extent of muscle contraction and 2) the muscle contraction
be maintained at a constant level using feedback methods. To correct VEMP ampli-
tudes, we use an average of rectified background muscle activity during a prestimu-
lus period of 20 ms [10] (Fig. 3). The corrected amplitude (CA) of VEMP is defined
as a ratio.
CA = (raw amplitude of p13–n23)/(mean background amplitude)
Fig. 1. Electrode placement for vestibular
evoked myogenic potential (VEMP)
recording
Fig. 2. Correlation between the VEMP
amplitude to 95-dBnHLtone bursts (500 Hz)
and mean background muscle activity in
healthy subjects. The correlation coefficient
was 0.83. dBnHL, dB normal hearing level;
0 dBnHL, average subjective threshold of
sound perception in healthy subjects. (from
Fig. 2 of ref. 24, Elsevier, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-30-320.jpg)
![Recording and Assessing VEMPs 27
For this purpose, one must average the rectified EMG during a prestimulus period.
A feedback method using a blood pressure manometer has been proposed to main-
tain the muscle contraction constant during VEMP recording [11].
To contract the SCM, we usually ask subjects in the supine position to raise
their head from the pillow. Alternatively, rotating the neck (in the supine position
or upright) or having the examiner push the patient’s forehead can be useful
(Fig. 4). The rotation method may be easier. However, investigators should note
that only unilateral responses are recordable, and muscle contraction becomes
easily asymmetrical when this method is applied. Head position itself does not
affect VEMP responses [12]. According to Isaacson et al., when the amplitude was
corrected according to tonic EMG activity, no significant difference was noted
among various test positions [13].
Fig. 3. Correction of amplitudes using rectified electromyography (EMG). Upper trace, unrecti-
fied response; lower trace, rectified response. Background muscle activities were calculated using
the shaded areas. VEMP amplitudes were corrected based on background muscle activity. (from
Fig. 1 of ref. 12, Taylor & Francis, with permission)
Fig. 4. Methods to contract the sternocleidomastoid muscle (SCM). a Position supine with the
head raised, b position sitting with the head turned away from the tested ear, c position sitting
with the head pushed against the finger to provide resistance](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-31-320.jpg)
![28 Vestibular Evoked Myogenic Potentials
Normal VEMP Responses
In healthy subjects, VEMP responses consist of initial positivity followed by nega-
tivity with short latencies. This biphasic response is termed p13–n23 after the peak
latency. In our clinic, the means ± SD of p13 and n23 were 11.8 ± 0.86 ms and
20.8 ± 2.2 ms, respectively (95-dBnHL clicks) [14]. Responses to STBs have 2- to
3-ms longer peak latencies [6]. These responses are clearly ipsilateral-dominant.
In other words, p13–n23 can be recorded on the SCM ipsilateral to the stimulated
ear, although p13–n23 on the contralateral side is absent or small (Fig. 5) [15].
Following p13–n23, later components (n34–p44) can be also observed (Fig. 6) [16].
Fig. 5. Laterality of VEMP responses.
VEMP responses (p13–n23) to 95-dBnHL
clicks were clearly ipsilateral-dominant.
(from Fig. 1 of ref. 15, Taylor & Francis,
with permission)
10 msec
100 m V
p13
n23
n34
p44
Fig. 6. Typical VEMP waveform in response to 95-dBnHL clicks in a healthy subject. Responses
are on the ipsilateral SCM to the stimulated ear](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-32-320.jpg)
![Recording and Assessing VEMPs 29
Later components are not of vestibular origin [1]. Amplitudes of responses
(p13–n23) depend on the degree of muscle contraction. In the ordinary situation,
the amplitudes range from 50 to 200 μV.
The polarity of the initial responses (positivity followed by negativity) implies
that this myogenic potential is caused by inhibitory inputs to the SCM [17]. This
finding is consistent with neurophysiological data from cats [18].
Parameters for Assessing VEMPs
The following parameters are used for clinical evaluation. In this section, VEMPs
refer to the early component (p13–n23).
Presence of VEMPs
VEMPs are usually present in healthy subjects, whereas some elderly subjects
exhibit an absence of response. This absence is considered pathological in subjects
<60 years of age (Fig. 7). In other words, absence of responses suggests dysfunc-
tion of the sacculocollic pathway. Even in older subjects, the unilateral absence of
responses is pathological. If an absence of VEMP responses is observed, an exam-
iner should first confirm that the headphone was adequately placed on the head.
Second, the examiner should determine if the subject has conductive hearing loss.
The absence of responses in patients with conductive hearing loss does not mean
dysfunction of the saccule or its afferents.
Fig. 7. Example of unilateral absence
of VEMP responses on the left side (L).
R, right side](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-33-320.jpg)
![30 Vestibular Evoked Myogenic Potentials
Interaural Difference of VEMP Amplitude
Side-to-side differences of VEMP amplitude can be expressed as percent VEMP
asymmetry [3].
%VEMP asymmetry = 100 |Ar − Al|/(Ar + Al)
where Ar and Al are the amplitudes of p13–n23 on the right and on the left; and
|Ar − Al| is the absolute value of Ar − Al.
When the affected side is already known, the percent VEMP asymmetry should
be calculated as follows.
%VEMP asymmetry = 100 (Au − Aa)/(Au + Aa)
where Au is the amplitude of p13–n23 on the unaffected side; and Aa is the ampli-
tude of p13–n23 on the affected side.
In our institution, we set the upper limit of percent VEMP asymmetry as 34.1
(mean +2 SD) in healthy subjects. When the percent VEMP asymmetry exceeds
34.1, the asymmetry is pathological. We usually regard the decreased side as
pathological unless there are other specific reasons for the abnormal result (Fig. 8).
Although a similar normal range was reported from another laboratory [19], the
normal limit should be set at each institution because recording conditions cannot
be totally identical.
Peak latency
Significant delay of peak latencies is also pathological. As the peak latency of p13
shows better reproducibility than that of n23, the peak latency of p13 is more
available clinically. Prolonged latencies are signs of retrolabyrinthine or central
Fig. 8. Example of unilaterally decreased
amplitudes on the left side](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-34-320.jpg)
![disorders (Fig. 9) [14, 20, 21]. In our institution, the means ± SD of p13 and n23
to 95 dBnHL click were 11.8 ± 0.86 ms and 20.8 ± 2.2 ms, respectively. The normal
range for peak latency should be established at each institution.
Threshold
In comparison with the auditory brainstem response (ABR), which is an evoked
potential of cochlear origin, the VEMP threshold is much higher. In our clinic, the
VEMP thresholds in healthy subjects (clicks) were ≥85 dBnHL. According to
Colebatch et al. [22], the mean threshold of VEMP responses to clicks in healthy
subjects was 86 dBnHL and the lowest was 70 dBnHL. When the threshold to click
stimulation is lower than 70 dBnHL, it is definitely pathological, suggesting hyper-
sensitivity of vestibular end-organs to sound (the Tullio phenomenon) (Fig. 10).
Fig. 9. Example of prolonged latencies
(both sides). (from Figure of ref. 20, BMJ
Publishing Group, with permission)
Fig. 10. Example of low VEMP thresh-
olds. In this subject, the threshold was
70 dBnHL
Recording and Assessing VEMPs 31](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-35-320.jpg)
![32 Vestibular Evoked Myogenic Potentials
Miscellaneous
In addition to the above-mentioned major parameters, some others can be applied.
Frequency Tuning Characteristics
Rauch et al. reported that patients with Meniere’s disease showed less tuning at
500 Hz and threshold elevation, whereas healthy subjects showed the best responses
at 500 Hz [23]. Their results suggested that a new parameter concerning frequency
tuning characteristics might be applicable for VEMP assessment. For example, the
ratio of corrected VEMP amplitude at 500 Hz to that at 1000 Hz or the ratio of the
threshold at 500 Hz to that at 1000 Hz might be applied.
Acoustic/Galvanic Ratio
The characteristics of the responses to acoustic stimuli and galvanic stimuli might
be compared. Because galvanic stimulation bypasses the labyrinth and stimulates
the vestibular nerve directly, labyrinthine damage does not affect galvanic VEMPs
whereas it does affect acoustic VEMPs. Therefore, the acoustic VEMP amplitude/
galvanic VEMP amplitude ratio can be a useful indicator for differentiating laby-
rinthine from retrolabyrinthine disorders [24–26]. This issue is discussed in the
chapter “VEMP Variants.”
References
1. Welgampola MS, Colebatch JG (2005) Characteristics and clinical applications of vestibular-
evoked myogenic potentials. Neurology 64:1682–1688
2. Bath AP, Harris N, McEwan J (1999) Effect of conductive hearing loss on the vestibulocollic
reflex. Clin Otolaryngol 24:181–183
3. Murofushi T, Matsuzaki M, Mizuno (1998) Vestibular evoked myogenic potentials in patients
with acoustic neuromas. Arch Otolaryngol Head Neck Surg 124:509–512
4. Sheykholeslami K, Murofushi T, Kaga K (2001) The effect of sternocleidomastoid electrode
location on VEMP. Auris Nasus Larynx 28:41–43
5. Endoh T, Hojoh K, Sohma H, et al (1987) Auditory postauricular responses in patients with
peripheral facial nerve palsy. Acta Otolaryngol Suppl 446:76–80
6. Murofushi T, Matsuzaki M, Wu CH (1999) Short tone burst-evoked myogenic potentials on
the sternocleidomastoid muscle. Arch Otolaryngol Head Neck Surg 125:660–664
7. Murofushi T, Curthoys IS, Topple AN, et al (1995) Responses of guinea pig primary vestibu-
lar neurons to clicks. Exp Brain Res 103:174–178
8. Murofushi T, Curthoys IS (1997) Physiological and anatomical study of click-sensitive
primary vestibular afferents in the guinea pig. Acta Otolaryngol (Stockh) 117:66–72
9. Wu CH, Murofushi T (1999) The effect of click repetition rate on vestibular evoked myogenic
potential. Acta Otolaryngol (Stockh) 119:29–32](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-36-320.jpg)

![VEMP Variants
Introduction
Originally, vestibular evoked myogenic potentials (VEMPs) were recorded on
cervical muscles, especially the sternocleidomastoid muscle (SCM), in regard
to air-conducted sound [1, 2]. However, there were some limitations of this test.
To overcome these limitations, variants of VEMP analysis have been proposed and
are classified into two categories: variants stimulation methods and recording
methods.
Variants of Stimulation Methods
In addition to conventional unilateral air-conducted sound stimulation, a binaural
simultaneous air-conducted sound stimulation method, a tapping method, a bone-
conducted sound method, and a galvanic stimulation method have been reported.
Binaural Simultaneous Stimulation Method
The binaural simultaneous air-conducted sound stimulation method was proposed
to reduce physical loading of subjects. During VEMP recording, subjects must keep
contracting the SCM, and it is sometimes difficult for elderly subjects to maintain
the contraction.
The saccular projection to the SCM is unilateral [3]. In other words, acoustic
stimulation to one ear affects only the SCM ipsilateral to the stimulated ear. In
healthy subjects, VEMPs are clearly ipsilateral-dominant [4]. Therefore, simultane-
ous binaural stimulation seems to enable sacculocollic reflexes on both sides at the
same time. Murofushi et al. compared VEMP responses of monaural click stimula-
tion with responses to binaural click stimulation in patients with unilateral peri-
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 34
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_5, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-38-320.jpg)
![VEMP Variants 35
pheral vestibular dysfunction [5]. In 26 of the 28 patients, results for monaural
stimulation coincided with those for binaural stimulation (Fig.1, Table 1). Among
the two patients without coincidence, one patient showed bilateral normal responses
to monaural stimulation despite decreased responses on one side to binaural simul-
taneous stimulation, and the other patient showed a unilateral absence to monaural
stimulation despite the bilateral absence of responses to binaural stimulation. The
reasons for the discordance were not clear. However, the high rate of coincidence
and no false-negative results encouraged us to apply the binaural simultaneous
stimulation method as a clinical screening test.
Tapping and Bone-Conducted Sound Method
Conduction problems in the external ear or middle ear attenuate the intensity of
air-conducted sound. Therefore, subjects with conductive hearing loss may show
an absence of response despite a normal sacculocollic pathway. The tapping and
bone-conducted sound method was primarily applied to measure vestibulocollic
Monaural stimulation
Binaural stimulation
Fig. 1. Vestibular evoked myogenic
potential (VEMP) responses to monau-
ral individual stimulation and binaural
simultaneous stimulation in a woman
with a left acoustic neuroma. The patient
showed absent responses on the left
sternocleidomastoid muscle (SCM) to
left ear stimulation and to binaural
stimulation. L, left side; R, right side.
(from Fig. 1 of ref. 5, Springer, with
permission)
Table 1. Results to unilateral stimulation and bilateral stimulation. (from Table 1 of ref. 5,
Springer, with permission)
Bilateral stimulation
Unilateral stimulation
Bilaterally
normal
Unilaterally
decreased
Unilaterally
absent
Bilaterally
absent Total
Bilaterally normal 9 0 0 0 9
Unilaterally decreased 1 0 0 0 1
Unilaterally absent 0 0 15 0 15
Bilaterally absent 0 0 1 2 3
Total 10 0 16 2 28](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-39-320.jpg)
![36 Vestibular Evoked Myogenic Potentials
reflexes in patients with conducted-hearing loss, as these stimulations are thought
to bypass the middle ear and act directly on the inner ear (Fig. 2).
The tapping method applied vibratory stimulation to the subjects’skulls. Tapping
with a tendon hammer evokes biphasic myogenic responses on the SCM as evoked
by air-conducted sound (Figs. 3, 4) [6–8]. The tapping site is the forehead (Fz) or
mastoid. Recording conditions are basically the same as for VEMP responses to
Air-conducted
sound
Bone-
conducted
sound,
tapping
Galvanic
stimuli
Fig. 2. Stimulation methods and supposed stimulated sites
Fig. 3. Tendon hammer that can send trigger signals to the averager](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-40-320.jpg)
![VEMP Variants 37
air-conducted sound. In a study of patients without conductive hearing loss, so long
as we concerned only if the results were normal 14 of 15 patients showed the same
results with the tapping method as were seen with the air-conducted click method.
However, many of them showed delayed positivity (Table 2). Although we had
regarded the delayed positivity as delayed p13, the positivity might be an inverted
response preceded by negativity (n13) (Fig. 5). There was no clear relation between
the responses evoked by tapping and pure-tone hearing. These findings suggested
that myogenic potentials evoked by tapping should be of vestibular origin but that
parts other than the saccule could be also stimulated. Brantberg and Mathiesen
reported that tapping-evoked myogenic potentials were preserved after resection
of the inferior vestibular nerve [9]. Their findings supported our assumption.
As described above, the tapping method is a good alternative to the conventional
VEMP procedure. One problem with the tapping method, though, was the difficulty
of calibrating the stimulation. The bone-conducted sound method overcomes
this defect. For recording VEMPs to bone-conducted sound, a bone vibrator—an
apparatus to measure pure-tone hearing thresholds—was first applied [10–14].
Currently, a bone vibrator with stronger intensity is available [15]. The bone
Fig. 4. VEMPs to 95-dBnHL clicks and myogenic responses to tapping in a healthy subject
Table 2. Results of myogenic potentials to clicks and tapping
Parameter Normal
Abnormal
Total
Decreased Absent Delayed
Normal 3 0 0 0 3
Abnormal
Decreased 1 0 0 1 2
Absent 0 0 4 5 9
Delayed 0 0 0 1 1
Total 4 0 4 7 15](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-41-320.jpg)
![38 Vestibular Evoked Myogenic Potentials
vibrator is placed on the mastoid or forehead. The other recording conditions
are basically the same as those for air-conducted sound. The optimal frequencies
for VEMPs to bone-conducted sound ranged from 200 to 250 Hz [14, 16]. As for
the tapping method, bone-conducted sound can stimulate not only the saccule but
other parts of the vestibular end-organs as well. A neurophysiological experiment
with guinea pigs suggested that the utricle could also be stimulated by bone-
conducted sound [17].
These methods—the tapping method and the bone-conducted sound method—
are suitable for assessing vestibular function of subjects with conductive hearing
loss [11–13]. One should note that these methods stimulate vestibular end-organs
on both sides. Whereas the saccular projection to the SCM is uncrossed, the utricu-
lar projection to the SCM is not only uncrossed but also crossed. Utricular stimula-
tion could have excitatory inputs to the contralateral SCM [3]. Therefore, we should
take the crossed pathway into account when assessing the results.
Galvanic Stimulation
Galvanic stimulation has been used for a galvanic body sway test and a galvanic
eye movement test [18–21]. Galvanic stimulation has been thought to bypass the
labyrinth and act directly on the vestibular nerve (Fig. 2) and thus is useful for
differentiating retrolabyrinthine lesions from labyrinthine lesions. Short-duration
galvanic stimulation has been also applied to patients with vestibular disorders for
this purpose [20–22]. That these potentials are of vestibular origin was confirmed
by the disappearance of myogenic potentials on the SCM after vestibular nerve
section [23].
Fig. 5. VEMP (95-dBnHL clicks) and myogenic responses to tapping in a woman with a left
acoustic neuroma. She showed absent responses to clicks and delayed responses to tapping.
Inverted responses?, small negativity might be an inverted response](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-42-320.jpg)
![VEMP Variants 39
The recording methods of myogenic potentials to galvanic stimuli are as follows.
The electrodes for stimulation were placed on the forehead (anode) and mastoid
(cathode), or on bilateral mastoids. The cathodal electrode should be placed on
the mastoid that an examiner wants to stimulate. The procedure is contraindicated
in patients who have an implanted electrical device such as a pacemaker or
a cochlear implant. It should also not be applied to patients who have medical
history of epilepsy.
A current of 3–4 mA (duration 1–2 ms) is used as the galvanic stimulus. Band-
pass filters, stimulation rate, and time window for analysis are the same as for
acquiring VEMPs to acoustic stimuli. Responses to 50–100 stimuli are averaged.
Galvanic stimulation produces huge electrical artifacts. To remove these artifacts,
the responses obtained without SCM contraction are subtracted from the responses
with SCM contraction [21, 22]. Using this subtraction method, we can get biphasic
(positive–negative) responses similar to VEMPs to acoustic stimuli (Fig. 6). We
call the first positivity p13g and the following negativity n23g. The means ± SD
of p13g and n23g with our method (3 mA, 1 ms) [21] were 11.4 ± 1.3 ms and 19.0
± 2.1 ms, respectively. The mean threshold was 2.5 mA. As these myogenic
potentials were abolished by vestibular nerve section [22], we call these myogenic
potentials to short-duration galvanic stimuli “galvanic VEMPs.”
As expected, patients with labyrinthine lesions such as Meniere’s disease have
normal galvanic VEMPs even though they had an absence of click VEMPs on
the affected side. In contrast, patients with retrolabyrinthine lesions (e.g., acoustic
neuroma) showed a tendency toward abnormal galvanic VEMPs in addition to
absent VEMPs in response to clicks [21]. This combined method of acoustic
p13g
n23g 10 msec
200 m V
100 m V
10 msec
With muscle contraction
Without muscle contraction
(a)
(b)
Fig. 6. Subtraction methods to make responses clearer. (from Fig. 1 of ref. 21, Elsevier, with
permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-43-320.jpg)
![40 Vestibular Evoked Myogenic Potentials
VEMPs and galvanic VEMPs has been applied for lesion site studies of various
diseases, as described in the clinical application chapters later in this book
[21, 24–27].
At an early stage, this combined method was applied only to patients with absent
VEMPs to acoustic stimuli. Recently, this combined method has been applied to
patients with preserved acoustic VEMPs. We can calculate the ratio of corrected
p13–n23 amplitudes to tone burst stimuli/corrected p13g–n23g amplitudes to gal-
vanic stimuli (the TG ratio). The TG ratio is significantly smaller on the affected
side of patients with endolymphatic hydrops (a representative inner ear disease)
than it is in healthy subjects or on the unaffected side of patients [28]. This change
in the TG ratio was not observed in patients with an acoustic neuroma. The
combined method of acoustic and galvanic VEMPs for lesion site studies is also
applicable to subjects with preserved acoustic VEMPs.
Variants of Recording Methods
Vestibular Evoked Extraocular Potential
Recently, myogenic potentials to acoustic stimuli recorded around the eyes—
vestibular evoked extraocular potential (oVEMP)—have been reported [15, 29, 30].
These responses consist of several peaks. The first peak is a negative deflection
with short tone bursts (mean 10.5 ms to 135-dBSPL, 500-Hz air-conducted bursts)
followed by a positive deflection (mean 15.9 ms) [30]. When air-conducted sounds
were presented, responses were contralateral eye-dominant and clearly recorded on
the electrodes placed underneath the lower eyelid [30] (Fig. 7, Table 3).
With our recording method [30], active electrodes are placed on the face
just inferior to each eye, with reference electrodes placed 1–2 cm below. Electro-
myography (EMG) signals are amplified and bandpass-filtered between 5 and
500 Hz. The time window for analysis is 50 ms. Although we prefer air-conducted
sound (500-Hz short tone burst, up to 135 dBSPL) because of unilateral stimula-
tion, others prefer bone-conducted sound or tapping because of clearer responses
[15, 29, 30]. Responses to 100 stimuli are averaged. Subjects are instructed to
maintain an upward gaze during recording because responses are the largest at this
gaze position.
It is believed that these responses reflect the vestibuloocular reflex, especially
the otolith-ocular reflex. The responses are called oVEMPs. Although a clinical
study suggested that oVEMPs are of vestibular origin, their exact origin is not clear
yet. The extent of the contribution of the saccule and utricule is controversial.
Further studies are required before this can be established as a definitive clinical
test. After establishing the neural pathway of oVEMPs, the combined use of
VEMPs and oVEMPs may be useful for assessing lesions in the brainstem, as
suggested by Rosengren et al. [31].](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-44-320.jpg)
![VEMP Variants 41
Fig.
7.
Vestibular
evoked
extraocular
potentials
(oVEMPs)
and
vestibular
evoked
myogenic
potentials
(cVEMPs)
to
500-Hz
tone
bursts
[135-dB
sound
pressure
level
(dBSPL)].
(from
Fig.
3
of
ref.
30,
Elsevier,
with
permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-45-320.jpg)
![42 Vestibular Evoked Myogenic Potentials
Neurogenic Potentials
For recording VEMPs (including oVEMPs), cooperation of the subject (maintain-
ing contraction of the SCM or an upward gaze) is essential. Therefore, it is difficult
to record VEMPs in children and elderly people and impossible in sleeping or
generally anesthetized subjects. If possible, vestibular evoked neurogenic potentials
(VENPs), which do not require muscle contraction, may be preferable. It was
reported that negativity at a latency of 3 ms (N3) was observed instead of normal
waveforms during auditory brainstem response (ABR) recording in subjects with
profound hearing loss [32]. Ochi and Ohashi hypothesized that N3 might be of
saccular origin [33]. As the presence of N3 corresponded to the presence of VEMPs
[34] (Fig. 8), N3 might be of saccule origin.
However, it was difficult to visualize N3 in subjects with normal hearing because
they have normal ABR waveforms (Fig. 9). To visualize N3 in normal subjects, it
is necessary to suppress or delete ABR waveforms. For this purpose, we presented
white noise to the stimulated ear, the intensity of which was high enough to stimu-
late the cochlea but insufficient for the vestibular nerve. We hypothesized that this
ipsilateral masking sound could suppress ABRs by disturbing the synchronization
of the cochlear nerve to the target sound but that it would not affect VEMPs. Based
on this hypothesis, we recorded N3 in subjects with preserved hearing under
white noise exposure (Fig. 10).We presented 1000-Hz short tone bursts (130 dBSPL,
rise/fall time 0.5 ms, plateau time 1 ms) as target stimuli with white noise
(100 dBSPL). The recording electrodes were placed on the vertex and mastoid.
Signals were amplified and bandpass-filtered (100–3000 Hz). Responses to 500
stimuli were averaged. The stimulation rate was 10 Hz, and the time window for
recording was 10 ms. Under these conditions, the mean latency of N3 of healthy
subjects was 3.58 ms, and the mean threshold was 125 dBSPL. The appearance of
N3 under these conditions corresponded well with the appearance of VEMPs [35].
We assume that the source of N3 might be around the vestibular nucleus.
Apart from N3, Todd et al. recorded potentials on the scalp evoked by bone-
conducted sound, the intensity of which was higher than the threshold of VEMPs
Table 3. Rate of identifiable responses, amplitude, and latency of oVEMP
Parameter
0.1-ms click
(135 dBSPL)
500-Hz short tone burst
(135 dBSPL)
Ipsilateral
eye
Contralateral
eye
Ipsilateral
eye
Contralateral
eye
Rate of identifiable responses
(% of 20 ears)
0 50 45 90
Amplitude between nI and
pI (μV)a
— 3.2 ± 0.4 1.9 ± 0.2 7.0 ± 1.0
nI latency (ms) — 8.8 ± 0.3 12.8 ± 0.6 10.5 ± 0.1
pI latency (ms) — 14.5 ± 0.5 17.7 ± 0.9 15.9 ± 0.3
(from Table 1 of ref. 30, Elsevier, with permission)
a
Mean ± SE](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-46-320.jpg)

![44 Vestibular Evoked Myogenic Potentials
[36]. In addition to auditory middle latency responses [37], the authors observed
positivity at about 10 ms (P10), which was maximum at Cz, and negativity at about
15 ms (N15), which was maximum at Fz. As P10 and N15 were also observed in
patients with bilateral profound hearing loss [38], these potentials are possibly
VENPs. The sources of these potentials and the origins at the end-organ level
should be clarified.
References
1. Colebatch JG, Halmagyi GM (1992) Vestibular evoked potentials in human neck muscles
before and after unilateral vestibular deafferentation. Neurology 42:1635–1636
2. Colebatch JG, Halmagyi GM, Skuse NF (1994) Myogenic potentials generated by a click-
evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry 57:190–197
3. Kushiro K, Zakir M, Ogawa Y, et al (1999) Saccular and utricular inputs to sternocleidomas-
toid motoneurons of decerebrate cat. Exp Brain Res 126:410–416
4. Murofushi T, Ochiai A, Ozeki H, et al (2004) Laterality of vestibular evoked myogenic
potentials. Int J Audiol 43:66–68
5. Murofushi T, Takai Y, Iwasaki S, et al (2005) VEMP recording by simultaneous binaural
stimulation. Eur Ach Otorhinolaryngol 262:864–867
6. Halmagyi GM, Yavor RA, Colebatch JG (1995) Tapping the head activates the vestibular
system: a new use for the clinical reflex hammer. Neurology 45:1927–1929
7. Murofushi T, Matsuzaki M, Ikehara Y, et al (2000) Myogenic potentials on the neck muscle
by tapping the head. In: Claussen CF, Haid T, Hofferberth B (eds) Equilibrium research,
clinical equilibriometry and modern treatment. Elsevier, Amsterdam, pp 233–238
8. Brantberg K, Tribukait A (2002) Vestibular evoked myogenic potentials in response to later-
ally directed skull taps. J Vestib Res 12:35–45
9. Brantberg K, Mathiesen T (2004) Preservation of tap vestibular evoked myogenic potentials
despite resection of the inferior vestibular nerve. J Vestib Res 14:347–351
10. Sheykholeslami K, Murofushi T, Kermany MH, et al (2000) Bone conducted evoked
myogenic potentials from the sternocleidomastoid muscle. Acta Otolaryngol (Stockh)
120:731–734
11. Monobe H, Murofushi T (2004) Vestibular neuritis in a child with otitis media with effusion;
clinical application of vestibular evoked myogenic potential by bone-conducted sound. Int J
Pediatr Otorhinolaryngol 68:1455–458
Fig. 10. N3 in a healthy subject with ipsi-
lateral white noise exposure. The shaded
area represents period of stimulation](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-48-320.jpg)



![Meniere’s Disease and Related Disorders:
Detection of Saccular Endolymphatic Hydrops
Introduction
Meniere’s disease (MD), a common inner ear disorder, is characterized by recurrent
vertigo attacks, fluctuating hearing loss, tinnitus, and a sensation of aural fullness
[1]. Guidelines for the diagnosis of MD have been published by the American
Academy of Otolaryngology, Head, and Neck Surgery (AAO-HNS) (Table 1) [2].
The incidence of MD varies from 21/100000 to 50/100000 [3–6]. MD develops
during middle age and shows a slight female predominance [7].
The combination of vertigo, hearing loss, and tinnitus were reported by Itard
(1821) [8]; and histopathological studies of the temporal bone in MD were pub-
lished in 1938 by Yamakawa [9] and Hallpike and Cairns [10]. They reported
endolymphatic hydrops in the temporal bone of MD patients at autopsy. According
to Schuknecht [11], during the early stage of the disease endolymphatic hydrops
involves principally the cochlear duct and the saccule. According to Okuno and
Sando [12], severe hydrops was observed in the saccule most frequently in their
histopathological study of the temporal bone. Therefore, a high incidence of abnor-
mal vestibular evoked myogenic potentials (VEMPs) in MD is expected.
Incidence of Abnormal VEMPs in Meniere’s Disease
In earlier studies, the incidence of abnormal VEMPs was 39% according to Muro-
fushi et al. [13] and 54% according to de Waele et al. [14]. We reviewed results of
VEMPs of MD patients in our clinic (n = 81; 32 men, 49 women; ages 16–75 years,
mean 50.7 years; 95-dBnHL clicks). Among the 81 patients, 39 (48%) had absent
VEMPs on the affected side; 8 patients showed decreased VEMP amplitude; and
34 patients had normal VEMPs (Fig. 1). Thus, the overall incidence of abnormal
VEMPs in MD patients was 58%.
Although MD patients showed absent or decreased VEMPs, they rarely dis-
played delayed peaks [15]. Rauch et al. reported an elevated VEMP threshold in
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 49
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_6, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-52-320.jpg)
![50 Vestibular Evoked Myogenic Potentials
MD patients and recommended that the threshold be a diagnostic parameter [16].
Rauch et al. also reported a shift of the best frequency of VEMP in MD patients.
According to previous studies, the best frequency of VEMP is between 300
and 700 Hz [17–19]. Whereas Rauch et al. reported that the best frequency is
500 Hz in healthy subjects, patients with MD showed less tuning at 500 Hz and
shifts of the best frequency to 1000 Hz [16]. We confirmed this tendency (Fig. 2).
Table 1. Dainostic scale for Meniere’s disease
Certain Meniere’s disease
Definite Meniere’s disease, plus histopathological confirmation
Definite Meniere’s disease
Two or more episodes of vertigo lasting at least 20 min
Audiometrically documented hearing loss on at least one occasion
Tinnitus or aural fullness
Probable Meniere’s disease
One definite episode of vertigo
Audiometrically documented hearing loss on at least one occasion
Tinnitus or aural fullness
Possible Meniere’s disease
Episodic vertigo without documented hearing loss
Sensorineural hearing loss—fluctuating or fixed—with disequilibrium but without definite
episodes
(from ref. 2)
In all scales, other causes must be excluded using any technical method
Absent
39 (48%)
Decreased
8 (10%)
Normal
33 (41%)
Increased
1 (1%)
Fig. 1. Vestibular evoked myogenic poten-
tial (VEMP) responses in Meniere’s disease
(MD) patients (n = 81)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-53-320.jpg)
![Meniere’s Disease: Detection of Hydrops 51
Rauch et al. attributed this shift to the change in resonant frequency in the saccule.
Comparison of the amplitudes and/or thresholds of VEMP in 500 Hz with those
in 1000 Hz could be a new parameter for VEMP that may be applicable to the
diagnosis of endolymphatic hydrops in the saccule. However, there have been
no reports concerning the specificity of this phenomenon. Thus, we need to
determine if this frequency shift is specific to Meniere’s disease or to endolym-
phatic hydrops.
Disease Stage and VEMPs
According to the guidelines of the AAO-HNS [2], the disease stage of MD is
determined by the average hearing level. Young et al. [19] studied VEMP results
in relation to the disease stage. Among 40 patients, 6 were classified as having
stage I MD. Five of the six patients showed normal VEMPs, and one had aug-
mented VEMPs on the affected side. Among the 12 patients classified as having
stage II MD, 7 had normal VEMPs, 2 had augmented VEMPs, 4 had decreased
VEMPs, and 2 had absent VEMPs. Among the 17 patients with stage III MD,
VEMPs were normal in 10, decreased in 4, and absent in 3. Among the five patients
classified as having stage IV MD, VEMPS were normal in two, decreased in one,
and absent in two. In that study, patients at advanced stages more frequently showed
absent or decreased VEMPs than patients at earlier stages.
Fig. 2. Frequency characteristics of VEMP responses in healthy subjects (n = 8). Among the
three tested frequencies (130 dBSPL), 500-Hz tone bursts tended to evoke the largest responses](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-54-320.jpg)
![52 Vestibular Evoked Myogenic Potentials
Proving Saccular Endolymphatic Hydrops Using VEMPs
Several clinical tests (e.g., electrocochleography, glycerol test, furosemide test)
have been utilized to provide evidence of endolymphatic hydrops [20–24].
Electrocochleography (ECochG) is a test of auditory evoked potentials, which
comprises potentials with short latencies (up to 2 ms). ECochG consists of
three components: cochlear microphonics (CM), summating potentials (SPs), and
compound action potentials (APs). The origin of CM and SPs is the cochlea,
whereas APs derive from the cochlear nerve. The (negative) SPs of MD patients
are significantly larger than those of healthy subjects [20, 21]. Such large SPs are
thought to reflect distention of the basilar membrane due to endolymphatic hydrops.
The ratio of the negative SPs to compound APs (CAPs) has been introduced as
a parameter. The upper limit of the normal range of the ratio SPs/CAPs has been
set at 0.30–0.40.
With the glycerol test, improvement of pure-tone hearing thresholds by glycerol
administration, an osmotic agent, has been thought to be caused by a temporary
reduction of endolymphatic hydrops in the cochlea [22, 23]. Endolymphatic hydrops
has been also reported to exist in the saccule [11, 12], although previous tests were
not able to prove it. Murofushi et al. [25], however, proposed the method to prove
the evidence of saccular hydrops in combination of VEMPs and glycerol adminis-
tration (glycerol VEMP test).
We record VEMPs prior to glycerol administration and 3 h after oral glycerol
administration (1.3 g/kg body weight) and measure the change ratio (CR) of the
VEMP (p13–n23) amplitudes.
CR (%) = 100(Aa − Ab)/(Aa + Ab)
where Aa is the p13–n23 amplitude 3 h after glycerol administration; and Ab is the
p13–n23 amplitude before glycerol administration.
The CR of six healthy volunteers was 3.52% ± 14.6% (mean ± SD). The normal
range was set at −25.7% to +32.7 % (within the mean ± 2 SD). Among the 17 MD
patients (4 men, 13 women; ages 24–72 years), 5 patients showed changes in VEMP
amplitudes exceeding the normal range (Fig. 3). Among the 17 patients, 10 had
abnormal VEMPs prior to glycerol administration. All the patients who showed
significantly large CRs had abnormal VEMPs prior to glycerol administration.
Therefore, 50% (5/10) of the patients with abnormal VEMPs showed significant
enlargement of VEMP amplitudes due to glycerol administration. Patients with
stage II MD most frequently had a positive glycerol VEMP test. The results of
glycerol VEMP testing were independent of the conventional glycerol test using
pure-tone audiometry. As glycerol VEMP testing can be performed simultaneously
with the conventional glycerol test, the glycerol VEMP test can provide supple-
mental information to detect endolymphatic hydrops [25, 26].](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-55-320.jpg)
![Meniere’s Disease: Detection of Hydrops 53
Drop Attacks in MD and VEMPs
In 1936, Tumarkin was the first to describe sudden drop attacks in patients with
MD [27]. Patients with MD who suffered from drop attacks suddenly felt a sensa-
tion of being pushed to the ground and then fell without loss of consciousness [28].
This phenomenon has been called Tumarkin’s otolithic crisis or a vestibular drop
attack (VDA) [3]. It has been thought that VDA occurred with sudden changes in
endolymphatic fluid pressure with inappropriate otolith stimulation causing reflex-
like vestibulospinal loss of postural tone. The abnormal bursts of neural impulses
from the otolithic organs would pass through the lateral vestibulospinal tract
(LVST), resulting in loss of postural tone. These hypothesized pathophysiological
mechanisms allowed us to assume that the function of the otolithic organ might
have some room to change when VDA occurs. In other words, the functions of the
otolithic end-organs may be unstable.
Ozeki et al. reviewed clinical records of 116 MD patients, finding 3 with VDA
[29]. Profiles of the three patients are summarized in Table 2. All three patients
showed recovery of VEMP responses spontaneously (patient 2) or after glycerol
administration (patients 1 and 3). These results imply that abnormal VEMPs in
such patients can be reversible and that their otolithic organs might be unstable.
After the bilateral VEMPs of patient 1 disappeared, there were no more drop
attacks. At the advanced stage—when bilateral otolithic organs were irreversibly
damaged—VDA may no longer occur.
Fig. 3. Effects of oral glycerol in a 69-year-old woman with left MD. She showed increased
VEMP amplitudes on the left side 3 h after glycerol administration. post-G, after glycerol; pre-G,
before glycerol; L, left side; R, right side. (from Fig. 1 of ref. 25, Elsevier, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-56-320.jpg)
![Meniere’s Disease: Detection of Hydrops 55
VEMPs in Delayed Endolymphatic Hydrops
Some people with unilateral profound hearing loss experience a delayed onset of
vertigo. Schuknecht called this condition delayed endolymphatic hydrops (DEH)
[30]. DEH can be subdivided into two types: ipsilateral and contralateral. Patients
diagnosed as having ipsilateral DEH have preexisting sensorineural hearing loss
in one ear and subsequent delayed-onset Meniere-type episodic vertigo without
fluctuating hearing loss in the other ear. In contrast, patients diagnosed as having
contralateral DEH have preexisting sensorineural hearing loss in one ear and
subsequent onset of fluctuating hearing loss in the other ear, with or without
Meniere-type episodic vertigo. According to Ohki et al. [31, 32], 67% (6/9) of
contralateral DEH patients and 75% (9/12) of ipsilateral DEH patients had abnor-
mal VEMPs (Table 3). Two of the four patients with ipsilateral DEH in their study
had positive glycerol VEMP testing results (Fig. 4). As patients with ipsilateral
DEH have profound sensorineural hearing loss on the affected side, it is difficult
to prove endolymphatic hydrops using ECochG or the conventional glycerol test.
However, with glycerol VEMP testing, it was possible to establish the presence of
saccular endolymphatic hydrops in patients with ipsilateral DEH. This is a signifi-
cant advantage of glycerol VEMP testing.
Fig. 4. Effects of oral glycerol on a 55-year-old woman with left-side ipsilateral delayed endo-
lymphatic hydrops. (from Fig. 1 of ref. 32, S. Karger AG, with permission)
Table 3. VEMP responses in patients with delayed endolymphatic hydrops
Type No.
No. with VEMP response
Normal Decreased Absent
Ipsilateral 12 3 5 4
Contralateral 9 3 0 6](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-58-320.jpg)
![56 Vestibular Evoked Myogenic Potentials
Galvanic VEMPs in Endolymphatic Hydrops
Delayed peaks of VEMP are signs of retrolabyrinthine or central lesions [15].
However, as the normal range of VEMP latencies is quite wide, it is difficult
to differentiate labyrinthine lesions from retrolabyrinthine lesions with only the
latencies of the peaks. Furthermore, in patients with absent VEMPs, we have no
information concerning latency. Hence, another method is required for the differ-
entiation of lesion sites.
Galvanic VEMP has been applied for this purpose [33–35]. As galvanic stimula-
tion bypasses the hair cells in the labyrinth and directly stimulates the distal portion
of the vestibular nerve, patients with absent acoustic VEMPs due to labyrinthine
lesions may have normal responses to galvanic stimuli. In fact, Murofushi et al.
showed that patients with absent acoustic VEMPs due to labyrinthine lesions
(Meniere’s disease or ipsilateral DEH) have normal galvanic VEMPs, whereas
almost all patients with absent acoustic VEMPs due to retrolabyrinthine lesions
(acoustic neuroma or other cerebellopontine angle tumor) showed absent or
decreased galvanic VEMPs (Fig. 5, Table 4) [35].
Furthermore, Murofushi et al. proposed methods to differentiate lesion sites
in patients with still-present VEMPs [36]. Murofushi et al. introduced the ratio of
the corrected p13–n23 amplitude to acoustic (tone bursts) stimuli/the corrected
amplitude p13g–n23g to galvanic stimuli.
Fig. 5. Relation of percent galvanic-evoked myogenic potential asymmetry to CP in the caloric
test. Almost all patients in group B (open circles) showed no or decreased responses to galvanic
stimulation on the affected side even though their caloric responses were preserved. In contrast,
group A patients (filled circles) showed normal responses to galvanic stimulation on the affected
side. GA, galvanic VEMP asymmetry; CP, canal paresis. Group A: Meniere’s disease or delayed
endolymphatic hydrops. Group B: acoustic neuroma or other cerebellopontine angle tumors. (from
Fig. 5 of ref. 35, Elsevier, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-59-320.jpg)
![Meniere’s Disease: Detection of Hydrops 57
TG (tone burst/galvanic) ratio = CA(p13–n23)/CA(p13g–n23g)
where CA(p13–n23) is the corrected amplitude of p13–n23 to tone bursts (500 Hz,
135 dBSPL); and CA(p13g–n23g) is the corrected amplitude of p13g–n23g to galvanic
stimuli (3 mA, 1 ms)
In 12 healthy volunteers, the TG ratio was 2.28 ± 0.66 (mean ± SD). In 12
patients with endolymphatic hydrops, the TG ratio was 1.16 ± 0.71. Among the 12
patients, 8 had a significantly small TG ratio (below the mean − 2 SD) (Fig. 6).
This tendency was not observed in patients with an acoustic neuroma.
The combined use of acoustic and galvanic VEMPs seems to be useful for dif-
ferentiating labyrinthine lesions from retrolabyrinthine lesions. However, de Waele
et al. reported that galvanic VEMPs could be abolished after intratympanic instil-
lation of gentamicin in MD patients [37]. Therefore, the diagnostic value of abnor-
mal galvanic VEMPs should be subjected to further study.
Fig. 6. Tone burst/galvanic ratio (TG) of healthy subjects and patients with endolymphatic
hydrops (EH). TG ratios of the affected side of patients were significantly smaller than those of
the unaffected side and in healthy subjects. (from Fig. 3 of ref. 36, Elsevier, with permission)
Table 4. Results of galvanic-evoked myogenic potentials in patients
Groupa
Increased Normal Decreased Absent Total
A 1 9 0 0 10
B 0 2 2 14 18
Total 1 11 2 14 28
a
Group A, Meniere’s disease or delayed endolymphatic hydrops; group B, acoustic neuroma or
other cerebellopontine angle tumors](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-60-320.jpg)


![Vestibular Neuritis (Neurolabyrinthitis) and
Other Peripheral Vestibulopathies:
Detection of Inferior Vestibular Nerve Damage
Introduction
Vestibular neuritis (vestibular neuronitis, vestibular neurolabyrinthitis, VN) is
characterized by prolonged severe vertigo with an acute onset. VN is not accom-
panied by cochlear symptoms or any other neurological symptoms [1, 2]. This
entity was described by Ruttin in 1909 [3] and by Nylen in 1924 [4]. It was
called vestibular neuronitis, and its symptomatology was summarized by Dix and
Hallpike [5]; but there is little evidence that the ganglion cells are primarily
inflamed.
The occurrence rate of VN in Japan was reported to be 3.5 per 100000 popula-
tion. The peak age at the time of the vertigo attack was during the fifth to sixth
decades [6].
Diagnostic criteria of VN are generally as follows.
• A single (or a few) vertigo attack(s) lasting for several hours to several days
• Severely damaged peripheral vestibular function usually proven by caloric
testing
• No cochlear signs or other neurological signs
Two causes have been proposed: viral inflammation and vascular disturbance.
Although there are only a few studies of the temporal bone pathology of VN, their
results have mainly supported the viral theory [7–9]. Of course, VN might be
caused by either of the two factors.
Vestibular Loss in VN: Complete or Partial?
Hypofunction of the peripheral vestibular system unaccompanied by cochlear or
other neurological signs must be demonstrated to establish a diagnosis of VN, as
mentioned above. Hypofunction of the peripheral vestibular system is usually
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 60
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_7, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-63-320.jpg)
![Vestibular Neuritis (Neurolabyrinthitis) 61
revealed by the caloric test or the head impulse test at the yaw axis [10]. These
tests mainly reflect functions of the vestibuloocular reflex, especially the lateral
semicircular canal-ocular reflex. Previous studies suggested that the vestibular loss
due to VN is in the superior vestibular nerve but that the inferior vestibular nerve
may be spared [11]. However, clinical tests of the peripheral vestibular system have
been focused on the lateral semicircular canal and its afferents, the superior ves-
tibular nerve. Furthermore, there are few samples of the temporal bone of the VN,
although the disease itself is common. The issue of involvement of the inferior
vestibular nerve remains to be clarified.
Recently, VEMP became a strong tool for evaluating the function of the saccule
and its main afferents, the inferior vestibular nerve. Murofushi et al. reported that
31 of 47 VN patients (66%) in Australia had VEMP responses on the affected side
but that 16 patients (34%) had an absence of VEMP responses [1]. Later, Murofushi
et al. performed a similar study in Japan. Among the 68 patients (40 men, 28
women; ages 18–78 years) 34 (50%) had normal VEMPs, whereas 31 (46%)
and 3 (4%) had absent and decreased VEMPs, respectively (Fig. 1). These results
imply that a considerable number of patients with VN could have deficits of
functions of the saccule and/or its afferents. Their results suggested that the VN
could be subdivided into the total VN and the superior VN (Fig. 2). Involvement
of the inferior vestibular nerve was also supported by a study on semicircular
canals [12].
Normal
34 (50%)
Absent
31 (46%)
Decreased
3 (4%)
Fig. 1. Vestibular evoked myogenic potential (VEMP) responses in 68 vestibular neuritis (VN)
patients](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-64-320.jpg)
![62 Vestibular Evoked Myogenic Potentials
Inferior VN
At this stage, one can assume that some dizzy patients might have a damaged
inferior vestibular nerve with a spared superior vestibular nerve. In other words,
patients could have inferior VN [13] (Fig. 3). As the inferior vestibular nerve is
an afferent of the posterior semicircular canal and the major part of the saccule,
a damaged inferior vestibular nerve could cause the signs and symptoms, as sum-
marized in Table 1.
We reviewed 1597 clinical records of the Balance Clinic at the University of
Tokyo Hospital [14]. Among the 812 patients who underwent both caloric tests and
VEMP tests, 4 patients fulfilled the inclusion criteria of possible inferior VN, as
shown in Table 2. During the same period, we had 30 patients with classic VN
(total VN and superior VN). Inferior VN might be less frequent than superior VN
Fig. 3. Inferior vestibular neuritis
Fig. 2. Classification of vestibular neuritis. a Superior vestibular neuritis, b total (superior and
inferior) vestibular neuritis](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-65-320.jpg)
![Vestibular Neuritis (Neurolabyrinthitis) 63
Table 1. Assumed clinical signs and symptoms of inferior
vestibular neuritis
Clinical symptoms
Sudden, severe, and prolonged vertigo
No auditory or other neurological symptoms
Clinical signs
Spontaneous tortional-vertical nystagmus
Postural imbalance
Posterior semicircular canal paresis
Paresis of the saccule
Preservation of superior vestibular nerve function
or total VN because of the anatomical differences in bony canals between the
superior vestibular nerve and the inferior vestibular nerve [15].
If the clinical entity of inferior VN were to be established, perhaps a clear diag-
nosis could be established in some patients whose diagnosis is in question.
Recovery of Vestibular Damage in VN
Recovery of equilibrium after a VN attack is achieved by vestibular compensation
and recovery of peripheral vestibular functions [16]. Concerning the recovery of
peripheral vestibular functions, the recovery of caloric responses (i.e., functions of
the lateral semicircular canal and its afferents) have been mainly reported. Accord-
ing to Okinaka et al. [17], caloric responses on the affected side of the VN recov-
ered to within the normal range in 37.2% at 2 years and in 50.0% at 5 years.
Concerning recovery of VEMP after a VN attack, Ochi et al. reported that
one of two VN patients with an absence of VEMP responses showed recovery of
responses to within the normal range at 15 months [18]. Murofushi et al. sequen-
tially recorded VEMPs in 13 patients with total VN. Four of these patients (30.7%)
showed recovery of VEMP responses to within the normal range at 2 years
(Figs. 4, 5), whereas only one patient (7.6%) showed recovery of caloric responses
[19]. These results imply that damage to the inferior vestibular nerve may be
reversed more quickly than that to the superior vestibular nerve in total VN.
Table 2. Inclusion criteria for possible inferior vestibular
neuritis
A single vertigo attack lasting at least several hours
Damaged saccular function shown as abnormal VEMPs
Normal caloric responses on both sides
No hearing loss or other neurological signs
Exclusion of other diseases
VEMP, vestibular evoked myogenic potentials](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-66-320.jpg)
![64 Vestibular Evoked Myogenic Potentials
Lesion Site of VN: Nerve or End-Organ?
Generally speaking, it has been believed that the VN lesion is in the vestibular
nerve. However, VN might be vestibular labyrinthitis.
To clarify the lesion site of VN, we recorded galvanic and click VEMPs in
patients with VN [20, 21]. Among those diagnosed as having total VN, patients
who showed an absence of caloric responses to ice water and an absence of click
VEMPs on the affected side (nine men, two women; ages 38–67 years) were
selected.Among them, eight (73%) showed an absence of galvanicVEMPresponses,
and three (27%) had normal galvanic VEMP responses (Fig. 6). These results sug-
gested that so-called vestibular neuritis could be vestibular labyrinthitis. The fact
that clinical VN could also have lesions in the labyrinth is appropriate for the
explanation of post-VN benign paroxysmal positional vertigo (BPPV). Although it
Fig. 4. VEMP recovery in a 51-year-old
man with left VN. a VEMPs 4 days after the
attack. b VEMPs 3 months after the attack.
c VEMPs 6 months after the attack. d
VEMPs 2 years after the attack. The patient
showed recovery of VEMP within 6 months,
and within 2 years his VEMPs were
normalized. L, left side; R, right side. (from
Fig. 2 of ref. 19, Taylor & Francis, with
permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-67-320.jpg)
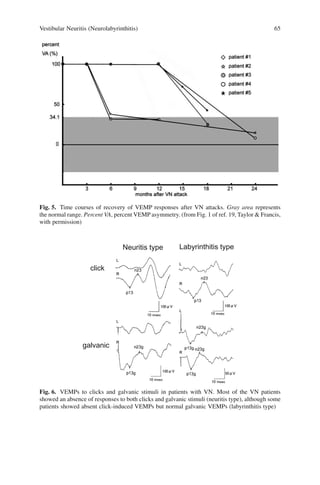
![66 Vestibular Evoked Myogenic Potentials
is well known that BPPV is frequently experienced after a severe vertigo attack of
VN [1], the reason for it is not clear. Inner ear damage due to vestibular labyrinthitis
might cause canalolithiasis if the posterior semicircular canal is preserved. Accord-
ing to Murofushi et al. [1], post-VN BPPV was observed in 10 of 47 VN patients
(21%). As VN could be vestibular labyrinthitis in 27% of the VN patients, it is
conceivable that the VN patients with vestibular labyrinthitis and preserved poste-
rior semicircular canal had post-VN BPPV.
Other Types of Peripheral Vestibulopathy
Ramsay Hunt Syndrome
Ramsay Hunt syndrome (RHS) is characterized by vestibulocochlear dysfunction
in addition to facial paralysis and auricular vesicles [22]. RHS is thought to be
caused by reactivation of a latent varicella-zoster virus (VZV) [23]. Although
Ramsay Hunt speculated that the infection of the eighth cranial nerve is spread
from the geniculate ganglion via a vestibulofacial anastomosis or a vestibuloco-
chlear anastomosis, there is the possibility that the VZV might arrive at the laby-
rinth through the oval and/or round window from a dehiscent facial nerve canal
[22–25]. In the latter case the lesion causing vestibular dysfunction could be in the
labyrinth, whereas in the former case it could be in the vestibular nerve.
Ozeki et al. applied combined methods of click and galvanic VEMPs to patients
with RHS [22]. Their results suggested that some patients have nerve lesions and
some have labyrinthine lesions (Fig. 7). In previous studies concerning auditory
dysfunction in RHS, lesion sites were retrocochlear in some cases and cochlear in
others [22, 26]. In other words, neurophysiological studies suggested that there
might be two routes by which the infection spread from the facial nerve to the
vestibulocochlear region.
Idiopathic Bilateral Vestibulopathy
Idiopathic bilateral vestibulopathy (IBV) is a clinical entity proposed by Baloh et
al. [27]. IBV represents bilateral dysfunction of the peripheral vestibular system
due to unknown causes. Patients with IBV complain of disequilibrium, vertigo, and
sometimes oscillopsia (Fig. 8). The following comprise some of the diagnostic
criteria for IBV.
• Bilateral decreased caloric responses (maximum slow phase eye velocity: 10°/s
or slower in the caloric test using ice water)
• No associated hearing loss
• Exclusion of bilateral vestibular dysfunction by known causes such as menin-
gitis or aminoglycoside ototoxicity, among others
• Exclusion of familial cases](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-69-320.jpg)

![68 Vestibular Evoked Myogenic Potentials
Baloh et al. classified IBV into two types: progressive and sequential. The pro-
gressive type is characterized by slowly progressive imbalance without episodic
vertigo, and the sequential type manifests as recurrent episodes of vertigo accom-
panied by persistent imbalance. We found a third type. Some patients diagnosed as
having IBV had a single vertigo attack and progressive imbalance. We called this
combination the one attack/progressive type. Among the 17 patients (10 men, 7
women; ages 33–75 years), 7 (41%) had the progressive type, 8 (47%) had the
sequential type, and 2 (12%) had the one attack/progressive type (Fig. 9). The one
attack/progressive type might be a transitional type from the progressive to the
sequential type.
In these 17 patients (34 ears), click VEMPs were bilaterally absent in 6 patients,
unilaterally absent in 6 patients, unilaterally decreased in 2 patients, and bilaterally
normal in 3 patients. In other words, 20 of the 34 sides (58%) showed abnormal
VEMPs. It means that 14 of the 17 patients (82%) had abnormal VEMP results.
These results suggested that the damaged area in patients with IBV could be limited
to the superior vestibular nerve region, although some patients showed damage to
both the superior and inferior vestibular nerve regions [28]. The study in a limited
number of patients suggested that the lesions were mainly in the vestibular nerve
[29]. Thus, IBV could be called vestibular neuropathy, which might be an auditory
neuropathy (auditory nerve disease) [30–33].
Superficial Siderosis
Superficial siderosis (SS) is a neurological disorder caused by the deposition of
hemosiderin in the central nervous system [34]. SS has been clinically char-
acterized as a combination of sensorineural hearing loss, cerebellar ataxia, and
pyramidal signs. Although it is known that 95% of SS patients have progressive
sensorineural hearing loss, there are few reports of the precise evaluation of
vestibular function of SS [35].
Progressive
7 (41%)
Sequential
8 (47%)
One attack and progressive
2 (12%)
Fig. 9. Classification of patients with IBV
according to the clinical course (sequential
or progressive)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-71-320.jpg)
![Vestibular Neuritis (Neurolabyrinthitis) 69
Ushio et al. reported a 64-year-old man with SS who had left total hearing loss
and right moderate sensorineural hearing loss. On T2-weighted magnetic resonance
imaging (MRI) scans, he showed linear hypointensity and signal losses in the cer-
ebellum, the medial surface of the temporal lobe, the midbrain, the pons, the spinal
cord, and the eighth cranial nerve (Fig. 10). Auditory brainstem response (ABR)
testing showed that he had no response on the left and only wave I on the right.
Caloric tests (ice water) did not show responses in either ear. He did not have
VEMP responses to either click (95 dBnHL) or galvanic (3 mA, 1 ms) stimulation
(Fig. 11). These results suggested that his vestibular loss as well as hearing loss
was caused by retrolabyrinthine lesions.
VEMP testing is also applicable to rare clinical entities such as SS.
Idiopathic Sudden Sensorineural Hearing Loss with Vertigo
Approximately 50% of the patients with idiopathic sudden sensorineural hearing
loss (ISSHL) could have vestibular symptoms [36], which may occur at the onset
of hearing loss or be delayed for hours or days. The histopathological study by
Schuknecht revealed atrophy of the saccular macula with hair cell loss, collapse of
the saccular wall, and distortion of the otolithic membrane in some temporal bones
but no abnormal findings in the utricular maculae and cristae [36]. Therefore, it is
conceivable that patients with ISSHL who have vertigo could display abnormal
VEMP responses.
Iwasaki et al. neurootologically studied 22 patients with ISSHL and vertigo.
Among them, 17 (77%) showed abnormal VEMP responses on the affected side,
and 10 (45%) had decreased caloric responses [37] (Fig. 12). The study by
(a) (b)
Fig. 10. Magnetic resonance imaging (MRI) findings from a 64-year-old man with superficial
siderosis. a T2-weighted, sagittal section. b T2-weighted, axial section. MRI scans showed linear
hypointensity around the cerebellum and brainstem (arrows). (from Fig. 3 of ref. 34, Taylor &
Francis, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-72-320.jpg)

![Vestibular Neuritis (Neurolabyrinthitis) 71
Iwasaki et al. [37] suggested that patients with ISSHL have damage in the saccule
more frequently than in the utricle or semicircular canals. Their findings are con-
sistent with histopathological studies. In fact, vestibular symptoms in patients with
ISSHL often manifest as unsteadiness. Vestibular symptoms in ISSHL patients
could be due to otolithic disorders. Galvanic VEMP study has supported the idea
that the lesion in ISSHL is in the labyrinth.
References
1. Murofushi T, Halmagyi GM, Yavor RA, et al (1996) Absent vestibular evoked potentials in
vestibular neurolabyrinthitis; an indicator of involvement of the inferior vestibular nerve?
Arch Otolaryngol Head Neck Surg 122:845–848
2. Strupp M, Brandt T (1999) Vestibular neuritis. Adv Otorhinolaryngol 55:111–136
3. Ruttin B (1909) Zur Differentialdiagnose der Labyrinth- und Hornerverkrankungen.
Z Ohrenheilkunde 57:327–333
4. Nylen CO (1924) Some cases of ocular nystagmus due to certain positions of the head. Acta
Otolaryngol (Stockh) 6:106–137
5. Dix MR, Hallpike CS (1952) The pathology, symptomatology, and diagnosis of certain
common disorders of the vestibular system. Ann Otol 61:987–991
6. Sekitani T, Imate Y, Noguchi T, et al (1993) Epidemiological survey by questionnaire in
Japan. Acta Otolaryngol (Stockh) 503:S9–S12
7. Schuknecht HF, Kitamura K (1981) Vestibular neuritis. Ann Otol 90(suppl 78):1–19
8. Nadol JB Jr (1995) Vestibular neuritis. Otolaryngol Head Neck Surg 112:162–172
9. Lyndsay JR, Hemenway WG (1956) Postural vertigo due to unilateral sudden partial loss of
vestibular function. Arch Otolaryngol 65:692–706
10. Halmagyi GM, Curthoys IS (1988) A clinical sign of canal paresis. Arch Neurol
45:737–739
11. Fetter M, Dichgans J (1996) Vestibular neuritis spares the inferior division of the vestibular
nerve. Brain 119:755–763
12. Aw ST, Fetter M, Cremer PD, et al (2001) Individual semicircular canal function in superior
and inferior vestibular neuritis. Neurology 57: 768–774
13. Halmagyi GM, Karlberg M, Curthoys IS, et al (2002) Inferior vestibular neuritis. Ann NY
Acad Sci 956:306–313
14. Iwasaki S, Takai Y, Ito K, et al (2005) Abnormal vestibular evoked myogenic potentials in
the presence of normal caloric responses. Otol Neurotol 26:1196–1199
15. Goebel JA, O’Mara W, Gianoli G (2001) Anatomic considerations in vestibular neuritis. Otol
Neurotol 22:512–518
16. Baloh RW (2003) Vestibular neuritis. N Engl J Med 348:1027–1032
17. Okinaka Y, Sekitani T, Okazaki H, et al (1993) Progress of caloric response of vestibular
neuritis. Acta Otolaryngol Suppl 503:18–22
18. Ochi K, Ohashi T, Watanabe S (2003) Vestibular-evoked myogenic potentials in patients
with unilateral vestibular neuritis: abnormal VEMP and its recovery. J Laryngol Otol
117:104–108
19. Murofushi T, Iwasaki S, Ushio M (2006) Recovery of vestibular evoked myogenic potentials
after a vertigo attack due to vestibular neuritis. Acta Otolaryngol 126:364–367
20. Murofushi T, Takegoshi H, Ohki M, et al (2002) Galvanic-evoked myogenic responses in
patients with an absence of click-evoked vestibulo-collic reflexes. Clin Neurophysiol
113:305–309
21. Murofushi T, Monobe H, Ozeki H, et al (2003) The site of lesions in “vestibular neuritis”:
study by galvanic VEMP. Neurology 61:417–418](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-74-320.jpg)

![Superior Canal Dehiscence Syndrome and
VEMPs: Detection of Hypersensitivity of the
Vestibular System to Sound
Introduction
Hypersensitivity of the vestibular system to sound is known as the Tullio phenom-
enon [1]. Clinically, the Tullio phenomenon has been reported in association with
various diseases, including congenital syphilis, congenital deafness, Meniere’s
disease, and perilymph fistula [2–5]. A fistula opening into the labyrinth (a third
window) and pathological contiguity of the tympanoossicular chain and the
membranous labyrinth have been assumed to be mechanisms of the Tullio phenom-
enon [3, 6].
Sound can induce nystagmus or ocular tilt reaction (skew deviation, ocular
torsion, head tilt) in patients with the Tullio phenomenon. Induced nystagmus can
be horizontal, vertical, torsional, or mixed [7, 8]. The most effective sound frequen-
cies to induce vestibular signs are between 500 and 1000 Hz [1, 7, 8].
According to Colebatch et al. [9], the mean threshold of vestibular evoked myo-
genic potential (VEMP) responses to clicks in healthy subjects was 86 dBnHL, and
the lowest was 70 dBnHL. The thresholds were lower in the ears of patients with
the Tullio phenomenon (<70 dBnHL) than in those of healthy subjects. Another
interesting point was a response on the contralateral sternocleidomastoid muscle
(SCM). In healthy subjects, there was no response on the contralateral SCM to
click stimulation [10], whereas patients with Tullio phenomenon showed negative–
positive responses with short latencies on the contralateral SCM. These contralateral
responses suggested that the utricule as well as the saccule could respond to clicks
on the affected ear because electrical stimulation of utricular afferents had excitatory
inputs to the contralateral SCM in addition to inhibitory inputs to the ipsilateral
SCM [11]. Large-amplitude VEMPs in patients with Tullio phenomenon could be
produced by the summation of responses derived from the saccule and utricule.
Superior Canal Dehiscence Syndrome
Superior canal dehiscence syndrome (SCDS) is a clinical entity introduced by
Minor et al. [5, 12]. This clinical syndrome results from dehiscence of bone overly-
ing the superior (anterior) semicircular canal and is characterized by vertigo or
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 73
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_8, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-76-320.jpg)
![74 Vestibular Evoked Myogenic Potentials
oscillopsia induced by pressure and/or a loud sound. SCDS is a newly established
entity that induces Tullio phenomenon and/or a positive fistula sign. Bone dehis-
cence of the superior semicircular canal can be revealed by high-resolution com-
puted tomography (CT) scans of the temporal bone (Fig. 1). Vertical-torsional
nystagmus or ocular tilt reaction is induced by pressure and/or a loud sound
[5, 13–15]. Compared with SCDS cases reported from the United States and
European Union countries, reports of SCDS in Japan are rare [16].
The prominent features of VEMPs in patients with SCDS are low-threshold,
high-amplitude responses to air-conducted sound [13, 15] (Fig. 2). These features
are consistent with hypersensitivity of the vestibular end-organs to sound in patients
with SCDS. Although this tendency was also observed with bone-conducted sound
Fig. 1. Coronal sections of computed tomography scans in a patient with superior canal dehis-
cence (SCD). This patient had bilateral dehiscence. R, right; L, left. (from Fig. 4 of ref. 16,
Igakushoin, with permission)
Fig. 2. Low thresholds of vestibular evoked myogenic potentials (VEMPs) in a patient with
bilateral SCD. (from Fig. 3 of ref. 16, Igakushoin, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-77-320.jpg)
![Superior Canal Dehiscence Syndrome 75
[17], galvanic VEMPs showed a normal threshold [18]. These findings suggested
that the hypersensitivity can be found in vestibular end-organs.
As eye movements induced by sound and/or pressure were considerably
decreased after plugging the superior semicircular canal [5], dehiscence in the
superior semicircular canal should cause deflection of the cupula in the crista
ampullaris of the superior semicircular canal by sound and/or pressure, resulting
in symptoms.
Patients with SCDS could have low-tone hearing loss with air–bone gaps at low
frequencies on pure-tone audiometry [19–21] (Fig. 3). Hence, it is important to dif-
ferentiate SCDS from otosclerosis. VEMPs are useful for this differential diagnosis
because patients with otosclerosis show clearly different responses. Patients with
otosclerosis have a high threshold of VEMPs in response to air-conducted sound.
The mechanism of air–bone gaps in SCDS has been thought to be the following:
the third window in the vestibular end-organs (dehiscence on the superior semicir-
cular canal) may shunt away acoustic energy from the cochlea to the vestibular
portions, resulting in hearing loss of air-conducted sound. In contrast, the presence
of the third window might result in more motion of fluid in the inner ear due to
bone vibration than the normal condition, leading to better bone-conducted hearing
[20, 22, 23]. In other words, air–bone gaps in patients with SCDS seem to be caused
by conduction problems in the inner ear; therefore, it should be called “pseudo-
conductive hearing loss.”
A similar air–bone gap was also reported in patients with large vestibular
aqueduct syndrome (LVAS) [24, 25]. The air–bone gap in LVAS might be analo-
gous to that in SCDS [23], although speculation that the air–bone gap in LVAS is
associated with restricted movement of the stapes was also proposed [25]. As LVAS
patients with an air–bone gap showed somewhat lower VEMP thresholds [26], the
Fig. 3. Air–bone gaps at low frequencies in SCD. O, hearing level to air-conducted sound;
<, hearing level to bone-conducted sound. (from Fig. 1 of ref. 21, Cambridge University Press,
with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-78-320.jpg)


![Migraine-Associated Vertigo and VEMPs:
Detection of Vestibular Signs in a Common
but Unclear Entity
Introduction
It has been recognized that migraine may cause recurrent vertigo/dizziness [1],
which is called migraine-associated vertigo (MAV) [2, 3]. The association
between migraine and vertigo has been supported by several epidemiological
studies [4, 5], and recently some diagnostic criteria for MAV have been proposed
(Table 1) [1, 3, 4]. MAV can be observed in patients with migraine without an aura
as well as migraine with an aura [6].
According to Iwasaki et al. [2], the average age of 33 patients diagnosed as
having MAV—based on the criteria of Brantberg et al. [3]—was 40 years (range
18–62 years), with a female predominance (23 women, 10 men). The median age
at onset of migraine was 27.5 years, and the median years predating the onset of
vertigo was 7.5 years. Of the 33 patients, 18 (55%) had migraine without an aura
and 15 (45%) had migraine with an aura. Rotational vertigo was noted in 24 of the
33 (73%) patients. Nonspecific dizziness and positional vertigo were also noted in
five and four patients, respectively. The duration of the vertigo attack ranged from
a few minutes to 3 days (Table 2). Most of the patients had vertigo attacks lasting
5 min to 24 h. Approximately 60% of the patients (20/33) had some cochlear symp-
toms during the attacks, among which bilateral tinnitus and bilateral aural fullness
were the most frequent (27% and 24%, respectively) (Table 3).
VEMPs and Other Neurootological Test Results
Canal paresis (CP) in caloric tests is observed in some patients with MAV. The
incidence of CP in previous reports ranged from 10% to 20% [2, 7, 8]. Horizontal
spontaneous nystagmus was also observed by electronystagmography in some
patients. Abnormal findings in pursuit, saccade, and the optokinetic nystagmus test
(which suggest central disorders) are rare in patients with MAV, whereas abnormal
findings suggesting central disorders are frequently observed in patients with
basilar-type migraine [9].
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 78
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_9, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-81-320.jpg)
![Migraine-Associated Vertigo 79
Table 1. Diagnostic criteria for migraine-associated vertigo (MAV)
Definite migrainous vertigo
Recurrent episodic vestibular symptoms of at least moderate severity
Current or previous history of migraine according to IHS criteria
One of the following migrainous symptoms during at least 2 vertiginous attacks
Migrainous headache
Photophobia
Phonophobia
Visual or other aura
Other causes ruled out by appropriate investigations
Probable migrainous vertigo
Recurrent episodic vestibular symptoms of at least moderate severity
One of the following
Current or previous history of migraine according to IHS criteria
Migrainous symptoms during at least two vertiginous attacks
Migraine-precipitants before vertigo for more than 50% of attacks (food triggers, sleep
irregularities, hormonal changes)
Response to migraine medications for more than 50% of attacks
Other causes ruled out by appropriate investigations
From Neuhauser et al. [5], Wiley-Blackwell, with permission
IHS, International Headache Society
Table 2. Duration and frequency of MAV attacks. (from ref. 2,
Taylor & Francis, with permission)
Parameter No.
Vertigo duration
<5 min 11 (33%)
5–60 min 8 (24%)
1–24 h 13 (39%)
>1 day 1 (3%)
Vertigo frequency
Times per day 6 (18%)
Times per week 17 (51%)
Times per month 5 (15%)
Times per year 5 (15%)
Table 3. Associated symptoms during vertigo attacks. (from
ref. 2, Taylor & Francis, with permission)
Associated symptoms No.
Cochlear symptoms 20 (61%)
Bilateral aural fullness 8 (24%)
Unilateral aural fullness 2 (6%)
Bilateral tinnitus 9 (27%)
Unilateral tinnitus 3 (9%)
Nausea 18 (54%)
Headache 16 (48%)
Vomiting 4 (12%)
Photophobia 3 (9%)
Phonophobia 2 (6%)
Other symptoms 3 (9%)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-82-320.jpg)
![80 Vestibular Evoked Myogenic Potentials
Similar to caloric tests, abnormal vestibular evoked myogenic potentials
(VEMPs) are seen in some patients. Iwasaki et al. [2] reported that 4 of the 33
patients (12%) showed absent VEMPs on either side. These four patients with
absent VEMPs were different from the four patients with CP on the caloric test.
These findings suggest that patients with MAV may have asymmetrical peripheral
vestibular hypofunction.
Liao and Young [10] studied 20 patients with basilar-type migraine (a special
form of migraine) [6]. Among the 20 patients, 10 had bilaterally normal responses,
4 had a unilateral absence of response, and 3 had bilaterally absent responses.
Furthermore, two patients showed bilaterally prolonged latencies, and one patient
showed a unilateral absence of responses and unilaterally prolonged latencies.
Patients with basilar-type migraine seem to have abnormal VEMPs more frequently
than the general MAV population. Moreover, the lesion sites of basilar type of
migraine may be in the central nervous system as well as the peripheral vestibular
system.
Migraine and Meniere’s Disease
As shown in previous studies [1–3], patients with MAV have cochlear symptoms
such as aural fullness and/or tinnitus and hypofunction in the peripheral vestibular
system. It has been reported that the prevalence of migraine was almost twice as
high in patients with Meniere’s disease (MD) as in the control group [11]. Ishiyama
et al. [12] reported that patients with MAV have vestibular drop attacks (Tumarkin’s
otolithic crisis), which has been considered a symptom of Meniere’s disease
[13–15].
These findings suggest that MAV shares some pathophysiology with MD.
Murofushi et al. compared frequency tuning in VEMPs of patients with MAV with
that of patients with MD. As reported by Rauch et al. [16], patients with MD
showed a tendency toward dominant VEMP responses to 1000-Hz tone bursts in
comparison with 500 Hz, whereas healthy subjects showed a tendency of dominant
responses to 500 Hz. A similar tendency was observed in patients with MAV [17].
This finding might support the existence of common pathophysiology between
MAV and MD.
Benign Recurrent (Paroxysmal) Vertigo of Childhood
The term “benign paroxysmal vertigo of childhood” was coined by Basser in 1964
[18]. This entity is defined as recurrent (five or more) attacks of severe vertigo that
resolve spontaneously after minutes to hours with normal neurological functions](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-83-320.jpg)
![Migraine-Associated Vertigo 81
and normal electroencephalography between attacks [6]. To represent this entity,
I prefer the term benign recurrent vertigo (BRV) to benign paroxysmal vertigo
because the latter can be confused with benign paroxysmal positional vertigo. BRV
has been considered a migraine equivalent or migraine precursor. BRV is a major
cause of vertigo in children [19, 20] (Fig. 1).
Ozeki et al. did not find clear peripheral vestibular weakness in patients with
BRV. Chang and Young found that 30% of the patients had abnormal caloric
responses, and 50% had abnormal VEMP responses. Vestibular functions in BRV
between and during attacks should be further studied [19, 21].
References
1. Neuhauser H, Lempert T (2004) Vertigo and dizziness related to migraine: a diagnostic
challenge. Cephalalgia 24:83–91
2. Iwasaki S, Ushio M, Chihara Y, et al (2007) Migraine-associated vertigo: clinical character-
istics of Japanese patients and effect of lomerizine, a calcium channel antagonist. Acta
Otolaryngol 559:S45–S49
3. Brantberg K, Trees N, Baloh RW (2005) Migraine-associated vertigo. Acta Otolaryngol
125:276–279
4. Furman JM, Marcus DA, Balaban CD (2003) Migrainous vertigo: development of a patho-
genic model and structured diagnostic interview. Curr Opin Neurol 16:5–13
5. Neuhauser H, Leopold M, von Brevern M, et al (2001) The interrelations of migraine, vertigo,
and migrainous vertigo. Neurology 56:436–441
Fig. 1. Causes of vertigo/dizziness in 38 children. BRV, benign recurrent vertigo; BPPV, benign
paroxysmal positional vertigo; VN, vestibular neuritis
BRV
8 (25%)
Psychogenic dizziness
2 (6%)
MD
1 (3%)
BPPV
3 (9%)
VN
1 (3%)
Miscellaneous
17 (53%)
N=32](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-84-320.jpg)

![Acoustic Neuroma and Other Cerebellopontine
Angle Tumors: Detecting a Neoplasm in the
Cerebellopontine Angle
Introduction
Acoustic neuromas (ANs) are schwannomas that arise mainly from the vestibular
divisionoftheeighthcranialnerve(vestibularnerve)[1].Theyconstitute6%ofintrac-
ranial tumors. In recent years, the incidence of newly diagnosed ANs in Denmark
was 13 per million population [2]. ANs are classified into two forms: sporadic and
neurofibromatosis type 2 (NF2). Sporadic ANs are usually unilateral, whereas
NF2 ANs, which are caused by a mutation of chromosome 22 [3], are usually
bilateral.
The most frequent symptoms of ANs are unilateral hearing loss and tinnitus [4]
(Table 1). Hearing loss is usually slowly progressive, although it may be of sudden
onset. The audiometric patterns are diverse (Fig. 1). Spinning vertigo is relatively
rare because the growth of the tumor is slow.
Diagnosis of Acoustic Neuroma
There are no exact guidelines for diagnosing AN. When patients have persistent
cochlear and/or vestibular symptoms not fully explained by another cause, AN
should be considered. Neurootological and audiological tests and neuro-imaging
studies are then required, including pure-tone audiometry, auditory brainstem
response (ABR) testing, vestibular evoked myogenic potential (VEMP) testing, and
the caloric test. Pure-tone audiometry is a screening test for hearing loss. ABRs,
VEMPs, and caloric tests are representative physiological tests of the cochlear
nerve, inferior vestibular nerve, and superior vestibular nerve, respectively. Mag-
netic resonance imaging (MRI) without contrast medium is primarily recommended
as a neuro-imaging study (Fig. 2).
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 83
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_10, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-86-320.jpg)
![84 Vestibular Evoked Myogenic Potentials
Sensitivity of VEMPs in Acoustic Neuroma
Abnormal findings of VEMPs in an AN patient are the absence of responses,
decreased amplitudes, and prolonged peak latencies of p13 and/or n23 on the
affected side. In our study, 39 of 62 AN patients showed an absence of responses
to 95-dBnHL clicks on the affected side, 9 showed decreased amplitudes, and 14
showed normal amplitudes [5]. In other words, 48 of the 62 patients (77%) had
abnormal findings concerning the amplitude. Among the 23 patients who exhibited
responses on the affected side, 4 (17%) had significantly prolonged peak latencies.
Table 1. Chief complaints of acoustic neuroma patients
Chief complaint No.
Slowly progressive hearing loss 63 (38%)
Sudden hearing loss 31 (19%)
Tinnitus 28 (17%)
Vertigo 14 (8%)
Dysequilibrium 9 (5%)
Miscellaneous 22 (13%)
Total 167 (100%)
Fig. 1. Pure-tone audiogram patterns in 65 acoustic neuroma (AN) patients
High tone loss
25
Cup-shaped loss
10
No hearing loss or
symmetric
7
Flat loss
7
Profound loss
6
Low tone loss
5
Dip-shaped loss
5](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-87-320.jpg)
![Acoustic Neuroma and Other Cerebellopontine Angle Tumors 85
R
Fig. 2. Magnetic resonance imaging (MRI) scan of a 48-year-old woman with a right AN
(white arrow). Her pure-tone hearing was normal in both ears
Fig. 3. Vestibular evoked myogenic potential (VEMP) responses in 62 AN patients. a Classifica-
tion according to amplitudes. b Classification according to latencies
Absent
a b
39 (63%)
Decreased
9 (14%)
Normal
14 (23%)
Prolonged
4 (17%)
Normal
19 (82%)
The overall sensitivity of VEMPs in these AN patients was 80% (50/62) (Fig. 3).
In the literature, the sensitivity of VEMPs in AN patients has been reported to
be 70%–80% [5–8]. Patko et al., who studied 170 patients with AN [7], reported
that 130 of the 170 patients (78.8%) showed abnormal VEMPs to click stimulation
and that the sensitivity was higher with click stimulation than with 500-Hz tone
burst stimulation.](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-88-320.jpg)
![86 Vestibular Evoked Myogenic Potentials
One must consider how to deal with subjects who showed bilateral absence of
VEMP responses to clicks. Recently, Ushio et al. studied the sensitivity of VEMPs
in AN patients with combined application of clicks and 500-Hz tone bursts [4].
They studied the sensitivity of VEMPs in 167 patients (79 men, 88 women) with
unilateral AN. When patients did not display VEMP responses to clicks on either
side, they were subjected to 500-Hz tone bursts. When the subjects exhibited
bilateral normal responses to 500-Hz tone bursts despite no responses to clicks,
they were regarded as being within the normal range. With this method, the sen-
sitivity was 81.6% for VEMPs, 80.2% for caloric tests, and 92.7% for ABRs. The
sensitivity of the ABR test results was significantly higher than that of the
VEMP results (P < 0.01) or the caloric test results (P < 0.01); whereas the sensi-
tivities of the VEMP studies and the caloric tests were not significantly different
(P = 0.76).
Concerning NF2 AN, Wang et al. [9] reported that only one of seven patients
showed abnormal VEMPs and speculated that NF2 ANs originated most often from
the superior vestibular nerve. However, as their study population was small, further
studies concerning VEMP sensitivity for NF2 ANs are required.
The sensitivity of VEMPs in AN patients was not as high as that of the ABRs.
However, some patients showed abnormal VEMP responses despite normal ABRs
[10] (Fig. 4). Furthermore, VEMP tests are applicable to patients with profound
sensorineural hearing loss, in whom ABR testing is not applicable. Because of
these merits, VEMPs should be included in the neurootological test battery of
AN patients.
Fig. 4. A 58-year-old woman with left AN who showed normal auditory brainstem responses
(ABRs) but absent VEMPs. The white arrow in the MRI scan (right) indicates a small tumor in
the internal auditory canal. (from Figs. 1 and 2 of ref. 10, Springer, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-89-320.jpg)
![Acoustic Neuroma and Other Cerebellopontine Angle Tumors 87
Tumor Size and VEMPs
According to Ushio et al., the tumors were significantly larger in patients with
abnormal caloric responses and in patients with abnormal ABRs. However, they
found no significant differences in tumor size between patients with normal and
abnormal VEMPs [4]. Murofushi et al. reported that prolonged peak latencies were
observed in patients with large tumors (>2 cm) (Fig. 5, Table 2) [5].
Lesion Site Detection Using VEMPs
Although absent VEMP responses and decreased VEMP amplitudes are usually
regarded as abnormal, these findings are not specific to ANs. In other words, these
Fig. 5. MRI scan of a large AN in a 54-year-old man
Table 2. Patients with acoustic neuroma and prolonged p13 and/or n23
No. Amplitude p13 (ms) n23 (ms) I–V (ABR) (ms) Tumor size (cm)
1 Normal 14.9 31.2 4.92 2
2 Decreased 14.6 21.2 Only wave I 3
3 Normal 14.4 27.4 5.20 2
4 Decreased 15.0 26.0 5.48 2
(from Table 1 of ref. 5, with permission. Copyright (2001) American Medical Association.
All rights reserved)
Upper limit of the normal range: p13, 13.5; n23, 25.2; I–V (ABR), interpeak intervals between
waves I and V of ABR: 4.4
Boldface values indicate prolonged latencies](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-90-320.jpg)
![88 Vestibular Evoked Myogenic Potentials
abnormal findings could be produced wherever a lesion is in the vestibulocollic
pathway.
The prolonged latency of p13 and/or n23 can be relatively specific in comparison
with amplitudes because prolonged latencies indicate a retrolabyrinthine lesion [5].
However, the incidence of prolonged latencies was not high (17%), and these laten-
cies were observed in patients with large tumors. It is one of the reasons that the
normal range of the peak latencies of VEMP is too wide to detect a small latency
prolongation.
Combined use of short-duration galvanic stimulation has been proposed as
another method to determine the lesion site [11]. Whereas relatively intense sounds
stimulate the vestibular end-organ, especially the saccular macula, galvanic stimuli
directly stimulate the vestibular nerve [12]. In fact, patients with Meniere’s disease
have normal VEMP responses to galvanic stimulation even though they show an
absence of VEMP responses to clicks. In contrast, most patients with ANs have an
abnormal galvanic VEMP response and an absence of VEMP responses to clicks
[11]. To apply this combined method to patients who have VEMP responses on the
affected side, Murofushi et al. introduced a ratio: corrected amplitude to acoustic
stimuli/corrected amplitude to galvanic stimuli [13]. They called it the TG ratio
(tone burst/galvanic ratio). As described in the chapter “Meniere’s Disease and
Related Disorders,” the TG ratio is significantly lower in patients with Meniere’s
disease than in healthy controls. In contrast, the TG ratio of patients with AN is
within the normal range or higher (Fig. 6). The combined use of acoustic and
galvanic VEMPs seems to be useful for determining a lesion site even in subjects
who display VEMP responses.
Fig. 6. Tone burst/galvanic (TG) ratio of patients with AN compared with those of healthy
subjects (Control) and patients with endolymphatic hydrops (Hydrops)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-91-320.jpg)
![Acoustic Neuroma and Other Cerebellopontine Angle Tumors 89
Tumor Origin and VEMPs
It is well known that ANs arise mainly from the vestibular nerve, which has two
divisions: superior and inferior. Some investigators reported almost equal origins
in the superior and inferior divisions, whereas others have reported a predominance
in the inferior branch [14, 15]. The caloric test mainly reflects the function of the
lateral semicircular canal and its afferents, and VEMP mainly reflects the function
of the saccule and its afferents. Therefore, one may expect that results of these two
tests can indicate the origin of tumors. However, so far we have not obtained posi-
tive results in regard to the correlation of test results and the origin of the tumor.
This may be because dysfunction can first emerge in a branch other than the branch
that is the origin of the tumor. This issue requires more careful study.
Other Cerebellopontine Angle Tumors and Lesions
The second most common tumor in the cerebellopontine angle (CPA) is meningi-
oma (Fig. 7) [16]. The rate of abnormal VEMPs in patients with a meningioma is
approximately 80%. Other masses in the CPA have also been reported, including
epidermoids, jugular foramen neurinomas, trigeminal neurinomas, arachnoid cysts,
chordomas, and metastatic tumors [17]. Occasionally, large aneurysms behave like
CPA masses (Fig. 8) [18, 19]. These masses can cause abnormal VEMPs when the
inferior vestibular nerve is involved. However, it is difficult to differentiate ANs
from other CPA masses using VEMP testing.
Fig. 7. MRI scan of a left-side cerebellopontine angle meningioma, coronal section](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-92-320.jpg)

![Disorders of the Central Nervous System and
VEMPs: Detecting Lesions in the
Vestibulospinal Pathway
Introduction
As vestibular evoked myogenic potential (VEMP) testing has been regarded as a
clinical test of the saccule and its afferents, it has been mainly applied to diseases
of the peripheral vestibular system. However, because the neural pathway of
VEMPs includes the vestibulospinal tract in the brainstem, it could also detect
disorders in the vestibulospinal tract, especially the medial vestibulospinal tract.
The application of VEMP testing to diseases that mainly affect the central nervous
system (CNS) are discussed in this chapter.
Multiple Sclerosis
Multiple sclerosis (MS) is the most common disease caused by an inflammatory
demyelinating process in the CNS. MS is characterized pathologically by multifo-
cal areas of demyelination with relative preservation of axons, resulting in much
reduced conduction velocity [1]. It is clinically characterized by dissemination of
signs and symptoms over space and time. McDonald et al. have proposed new
diagnostic criteria for MS [2].
Evoked potentials such as auditory brainstem responses (ABRs), somatosensory
evoked potentials (SEPs), and motor evoked potentials by transcranial magnetic
stimulation, among others, have been utilized in the clinical setting [3, 4]. As it has
been reported that vertigo is a symptom in 30%–50% of MS patients, it is expected
that VEMP testing in MS patients may reveal abnormal findings. Shimizu et al.
[5] first reported VEMP results in three patients with definite MS. All three
patients had high-intensity areas on T2-weighted magnetic resonance imaging
(MRI) in the areas involving the vestibulospinal tract and prolonged peak latencies
of VEMPs (Fig. 1). Later, Murofushi et al. reported that all of their six patients
with MS had abnormal VEMPs (prolonged latencies or absent responses) [6].
These patients had disequilibrium and/or vertigo as symptoms. Therefore, the
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 92
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_11, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-95-320.jpg)
![Detecting Lesions in the Vestibulospinal Pathway 93
sensitivity of VEMP testing in overall patients with MS remains to be clarified.
According to Versino et al. [7], abnormal VEMP responses were seen in 31% of
MS patients; and Bandini et al. [8] reported prolonged p13 latencies in 42% of MS
patients. This rate was much higher in patients with a history or signs/symptoms
of brainstem dysfunction.
With all the studies of VEMPs in MS, the main abnormal finding was prolonga-
tion of the peak latencies. This point distinguishes MS from other diseases with
peripheral disorder. VEMP testing is a valuable tool for detecting demyelination in
the vestibulospinal tract of MS patients.
Recently, in addition to VEMPs in cervical muscle (cVEMP), VEMPs around
the eye (oVEMP) have been recorded. cVEMPs reflect the function of the vestibu-
locollic reflex, and oVEMPs seem to reflect that of the vestibuloocular reflex. The
combined use of cVEMPs and oVEMPs might be useful for determining the site
of the lesion in the brainstem [9].
Spinocerebellar Degeneration
Spinocerebellar degeneration, one of the clinical entities causing disorders of the
central vestibular system, is characterized by cerebellar and/or spinal ataxia. It
Fig. 1. Magnetic resonance imaging (MRI) (left) and vestibular evoked myogenic potential
(VEMP) (right) findings from a 30-year-old woman diagnosed with multiple sclerosis. (from
Figure of ref. 5, BMJ Publishing Group, with permission)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-96-320.jpg)
![94 Vestibular Evoked Myogenic Potentials
consists of a sporadic type and a hereditary type. The hereditary type contains
autosomal recessive ataxias such as Friedreich’s ataxia and autosomal dominant
ataxias such as spinocerebellar ataxia-1 (SCA1) [10].
There are only a few reported studies concerning VEMPs in spinocerebellar
degeneration. Takegoshi and Murofushi studied VEMPs (click stimulation) in
patients with spinocerebellar degeneration [11]. Patients with olivopontocerebellar
atrophy and cerebellar cortex atrophy had normal VEMP responses on both sides.
In contrast, patients with Machado-Joseph disease (MJD, SCA3) showed an absence
of responses and prolonged latencies. Among the five patients with MJD in whom
we recorded VEMPs (including the three patients reported by Takegoshi and
Murofushi), one patient showed normal responses on both sides, whereas the
other four patients showed an absence of responses or prolonged latencies at
the first recording (Fig. 2). A 63-year-old man with MJD had prolongation of
latencies on both sides at the first recording, and 1 year later these responses
were abolished (Fig. 3). The progression of the disease is reflected in the VEMP
responses. The site of the lesion causing abnormal VEMPs in MJD remains unclear.
It is known that patients with MJD show an absence of caloric responses at the
early stage of the disease [12, 13] and that peripheral sensory nerves can be
involved in MJD [14]. Therefore, it is most likely that abnormal VEMPs in MJD
may be attributed to lesions in the vestibular nerve. However, lesions in the
vestibulospinal tract and in the labyrinth might have some effect. More studies
are required concerning VEMPs in patients with spinocerebellar degeneration,
including MJD.
Normal
3
Prolonged
4
Absent
3
Fig. 2. VEMP responses of patients with Machado-Joseph disease (n = 10 sides of five
patients)](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-97-320.jpg)
![Detecting Lesions in the Vestibulospinal Pathway 95
Brainstem Infarction
A vascular accident in the brain can cause damage to vestibular nerve fibers, ves-
tibular nuclei, and the vestibulospinal tract. These substrates are involved in the
neural pathway of VEMPs, and patients with a brainstem infarction may show
abnormal VEMPs. So far, reports of VEMPs in patients with brainstem/cerebellar
infarction are scarce [15–17]. Itoh et al. reported that patients with upper brainstem
infarction had normal VEMPs and abnormal ABRs, and patients with infarction in
the middle brainstem or the lower brainstem have abnormal VEMPs. Patients with
middle brainstem infarction have abnormal ABRs, whereas those with lower brain-
stem infarction have normal ABRs [15].
Wallenberg syndrome (WS), or lateral medullary infarction, is a clinical entity
that involves the vestibular nuclei (Fig. 4). WS is caused by occlusion of the ver-
tebral artery or arteries arising from the vertebral artery [18]. Dieterich and Brandt
reported ocular motor abnormalities in 36 patients with WS [19]. Almost all of the
patients exhibited gaze-evoked nystagmus and lateropulsion of the closed eyes, and
16 of the 36 patients had skew deviation (hypotropia) of the ipsilateral eye. In the
report by Itoh et al. [15], VEMPs were normal in some patients. This result is not
surprising because WS does not always involve the entire area of the vestibular
Fig. 3. VEMPs (95-dBnHL clicks) from a man with Machado-Joseph disease. At the first record-
ing, he showed bilateral prolonged latencies on both sides. One year later, he showed no response.
L, left side; R, right side. MRI showed atrophy of the pons and cerebellum](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-98-320.jpg)



![Neurootological Application of VEMP
Recording During Infancy and Childhood
Introduction
Vestibular evoked myogenic potential (VEMP) recording is a new tool for explor-
ing the pathways of the sacculus, inferior vestibular nerve, vestibular nucleus, and
sternocleidomastoid muscles (SCMs) in pediatric otology and neurotology.
Child Development and VEMPs
There have been few studies of the feasibility of recording VEMPs in infants and
small children, much less the characteristics of this response in neonates. Although
the muscle tone of neonates and young infants is poor compared with that of grown
children and adults, it is possible to record VEMPs from the SCM during infancy
and early childhood. Sheykholeslami et al. [1] reported that reproducible biphasic
VEMPs are recorded from the SCM of all the infants they examined (12 healthy
infants and children, ages 1–12 months) using loud and short-tone burst sounds.
Typical developmental changes in VEMPs in infants and children are shown in
Fig. 1 [2]. In these normal infants and children, air-conducted sound evoked a
biphasic response (p13 and n23 peaks) of VEMPs that were of larger amplitude
and shorter latency than those in adults. The difference in VEMPs on the side of
the stimulated ear is due to developmental changes in the distance of the pathway
between the sacculus and the SCM and changes in the strength of muscles. However,
neonatal VEMPs varied in amplitude, with consistent timing for peak p13 but
shorter peak n23 latencies than those in adult VEMPs.
The difficulties we encountered during VEMP recording in this study were as
follows: 1) We had difficulty maintaining the desired electromyographic (EMG)
activity of the SCM during the period of data acquisition, which required several
interruptions of the recording session and restarting data collection after achieving
the same level of muscle contraction. 2) The recording sessions were much longer
for infants partly because of the difficulty mentioned above and partly because of
the time necessary to educate parents and have their help during the test. 3) Finally,
it was necessary to have a technician in the room to position patients and control
the muscle contraction level [1].
Vestibular Evoked Myogenic Potential: Its Basics and Clinical Applications. 101
Toshihisa Murofushi and Kimitaka Kaga
doi: 10.1007/978-4-431-85908-6_12, © Springer 2009](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-102-320.jpg)
![102 Vestibular Evoked Myogenic Potentials
Incidence of Normal VEMPs in Infants with Congenital
Profound Hearing Loss
Can air-conducted sound-induced VEMPs be recorded even in infants with con-
genital profound hearing loss? This is an important question. It has been demon-
strated that loud clicks generate short-latency VEMPs [3] in normal adults.
Pathological human models have also been used to provide further evidence of the
vestibular (saccular) origin of the potentials. Colebatch et al. [3] showed that
VEMPs were evident at a high incidence in patients with profound sensorineural
hearing loss, but that they were abolished in all of their patients who underwent
unilateral vestibular neurectomy. These authors also reported that VEMPs were
abolished in some but not all patients with unilateral loss of a caloric response after
vestibular neuritis. They hypothesized that VEMPs are of vestibular origin and that
the saccule is probably an acoustically sensitive organ.
In our study, 67% of the 54 ears of 33 children with congenital profound hearing
loss showed normal VEMPs (Fig. 2), but 5% of ears of the children showed abnor-
mal VEMPs with low amplitude. This is a surprising finding because although they
cannot hear air-conducted loud click stimuli at all they have VEMPs, suggesting
that VEMP testing may be a new tool to illuminate vestibular activity in deaf infants
and children.
Fig. 1. Typical developmental changes in vestibular evoked myogenic potentials (VEMPs) in
normal infants and children](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-103-320.jpg)
![Pediatric Neurootological Application of VEMPs 103
Sheykholeslami et al. [4, 5] confirmed the saccular origin of this short-latency
acoustic response and that a saccular acoustic response persists in the human ear
and has a well-defined frequency tuning curve. Currently, recorded VEMPs are
induced using various stimuli including clicks [3], tone bursts [4], electrical stimuli
[6], bone-conducted sounds [7], and head taps [8]. VEMPs are gaining more atten-
tion as a diagnostic and prognostic test for otological and neurotological disorders
in infants and children as well as adults.
VEMPs and Inner Ear Anomalies
Evaluation of residual auditory and vestibular function in patients with inner ear
anomalies is a challenging issue in otology and neurotology because of the lack
of appropriate tests. Computed tomography (CT) and MRI studies of the temporal
bone cannot confirm the types of inner ear anomaly; they can only identify the
affected sensory organ and primary afferent nerve in the presence of an inner ear
anomaly. Therefore, the promontory test for electroneural hearing, the caloric
test, and the rotation chair test for semicircular canal function are performed.
VEMP testing is thus a new promising tool for examining sacculovestibular
nerve function.
Sheykholeslami and Kaga studied VEMPs in seven children with inner ear
anomalies, including two with cystic cochleas, three with shortened cochleas, and
two with completely absent cochleas [4]. The horizontal semicircular canals were
cystic in two patients and absent in five. Posterior semicircular canals were normal
Fig. 2. Click-evoked normal VEMPs (right) and audiogram (left) of a child with congenital
profound hearing loss](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-104-320.jpg)

![Pediatric Neurootological Application of VEMPs 105
Fig. 4. Bottom Click-evoked VEMPs (right) and audiogram (left) of a child with a large vestibu-
lar aqueduct. Top High-resolution computed tomography and magnetic resonance image of the
large vestibular aqueduct of the temporal bone
vestibules and inferior vestibular nerves are preserved in patients with these inner
ear anomalies. This study supports previous findings that VEMPs in humans have
their origin in the inner ear vestibules.
VEMPs and a Large Vestibular Aqueduct
Vestibular aqueduct (VA) enlargement is a distinct clinical syndrome in the spec-
trum of congenital inner ear anomalies. The bony anomaly of the enlarged VA
renders the membranous labyrinth vulnerable to sudden fluctuation in pressure,
which leads to progression of sensory neural hearing loss after head trauma.
Sheykholeslami et al. reported that VEMPs in three patients with a large ves-
tibular aqueduct (LVA) had greater amplitude and lower threshold and that the
vestibular organ was more responsive to sound and inner ear pressure changes [9].
Figure 4 shows a high-resolution CT image of LVA of the temporal bone and](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-106-320.jpg)
![106 Vestibular Evoked Myogenic Potentials
VEMPs in a child with profound deafness. These authors speculated that abnormal
transmission of inner ear volume and pressure to the cerebrospinal fluid space
via the widely patent VA and a large deflection of vestibular sensors in the mem-
branous labyrinth in response to stapes movements are possible contributors to
the generation of high-amplitude, low-threshold VEMPs in patients with LVA.
LVA is considered to be the third window, following the oval and round windows,
in the labyrinth.
VEMPs in Children with Cochlear Implants
The cochlear function of both ears is markedly impaired in infants and children
who are candidates for cochlear implantation. However, vestibular function is
also impaired in 10%–20% of such infants. After cochlear implantation, patients
can hear speech sounds, which are converted to electrical signals in a speech pro-
cessor; these signals are transmitted to the internal receiver under the scalp and
conducted to the electrodes in the cochlea. Thus, cochlear nerves that are stimulated
electrically convey information to the central auditory brainstem pathway and
auditory cortex.
There are two problems for vestibular end-organs after cochlear implantation.
One is traumatic damage of vestibular end-organs incurred following insertion of
the electrodes of the cochlear implant into the scala tympani. Tien and Linthicum
histopathologically analyzed the vestibular apparatus from human temporal bones
after cochlear implantation was carried out [10]. They observed significant damage
to the vestibular end-organ in approximately half of the temporal bones. Fibrosis
of the vestibular apparatus, and osteogenesis neurons were observed in all their
patients [10]. The other problem is that electrical stimulation may affect not only
the cochlear nerve but also the facial nerve or the vestibular nerve in patients with
a multichannel cochlear implant because of current spread. Based on these findings
VEMPs are considered useful for evaluating electrical current spread to the inferior
vestibular nerve.
Jin et al. compared VEMPs before and after surgery [11]. Before surgery, 6 of
the 12 children showed normal VEMPs, 1 showed a decrease in the amplitude of
VEMPs, and 5 showed no VEMPs.After surgery, with the cochlear implant switched
off, 11 showed no VEMPs and one showed decreased VEMPs. These results reveal
that even normal VEMPs disappear owing to trauma following electrode insertion.
With the cochlear implant switched on, four children showed normal VEMPs, but
eight did not (Fig. 5). This can be explained by the fact that these four children’s
inferior vestibular nerves were stimulated by the spread of electrical current from
the cochlear implant. We questioned why one-third of these children with cochlear
implants showed VEMPs but others did not. Later, Jin et al. demonstrated that
VEMPs evoked by cochlear implants may be related to an electrical current inten-
sity at a comfortable level (C level), particularly in channels that are closer to the
apical turn of the cochlea [12].](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-107-320.jpg)
![Pediatric Neurootological Application of VEMPs 107
Fig. 5. Changes in VEMPs before and after cochlear implantation. a Before surgery. b Switched-
off cochlear implant (CI) after surgery and switched-on CI after surgery
The patients who showed no VEMPs with the cochlear implant switched on may
require higher current intensities to elicit clear VEMPs (if they need to be recorded).
However, it is difficult to increase current intensity in such children because they
feel pain or facial nerve stimulation when the current intensity is higher than the
C level.
VEMPs of Pediatric Patients with Neurological Diseases
VEMPs depend on the higher tonus of the SCM. During VEMP recording, the
children were instructed to lift their heads or to turn their heads to the contralateral
side to induce hypertonicity in the SCM. In patients who showed hyper- or hypo-
tonicity of cervical muscles (e.g., those with cerebral palsy or other forms of severe
brain damage characterized by paralysis of extremities), it was not possible to
record VEMPs owing to the lack of muscle contraction even when the sacculoin-
ferior vestibular nerve was normal. In Fig. 6, VEMPs of a 3-year-old boy who has
cerebral palsy due to Pelizaeus-Merzbacher disease are absent, but the auditory
brainstem responses show waves I and II only [13]. VEMPs may thus be used as
an objective indicator of hyper- or hypotonicity of muscles in these neurological
diseases.](https://image.slidesharecdn.com/vemp-kaga-230911091840-210954b0/85/VEMP-KAGA-pdf-108-320.jpg)



