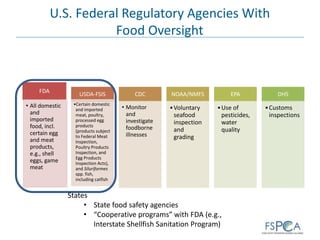
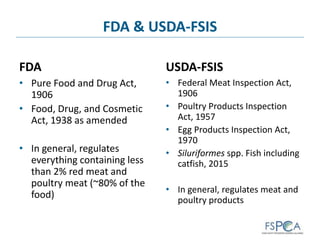
The document provides background information on the U.S. food safety system. It discusses the main federal regulatory agencies that oversee food safety - the USDA and FDA. The USDA regulates meat and poultry products while the FDA regulates all other foods, including imports. Other agencies like CDC and EPA also play roles. The agencies have different statutory authorities that influence their oversight approaches. The U.S. system relies on cooperation between federal, state, and local authorities.