This document contains formulas and concepts related to thermodynamics. It defines formulas for changes in internal energy, work, enthalpy, entropy, Gibbs free energy, and reaction enthalpy and entropy. It also provides examples of numerical problems calculating thermodynamic quantities like heat, enthalpy change, entropy change, and temperature or reaction spontaneity based on given values and thermodynamic formulas.


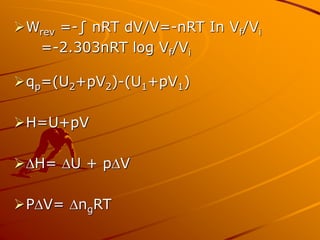






![Calculate the enthalpy change on freezing of
1.0 mol of water at -10 C to ice at-10C
.∆fus=6.03kJ /mol at 0 C. Cp[H2o(l)]=75.3J
/mol/k :Cp[H2o(s)]=36.8J /mol/K .
Sol. The freezing process is represented as
H2O(l) H2O(s) T1=-10 C =263.15K;
T2=0 C=273.15K;∆T=T2-T1=10K
Now according to Kirchoff’s equation ,
(∆H2-∆H1)/T2-T1=Cp (ice)-Cp (water)
(-6030-∆H1)/10=36.8-75.3
-6030-∆H1=10*38.5
∆H1= 5625J /mol](https://image.slidesharecdn.com/unit-6-thermodynamics-220730192944-61c31657/85/unit-6-thermodynamics-ppt-10-320.jpg)



![Fir the reaction;2A(g)+B(g) 2D(g)
∆U298=-10.5kJ and ∆S=-44.1J
Calculate ∆G298 for the reaction and predict
whether the reaction is spontaneous or not.
Sol. ∆H= ∆U+ ∆n(g)RT
∆ n(g)=2- ∆ 3=-1mol;T=298K; U=10.5kJ
R=8.314kJ/K/mol
∆H=-10.5+[-1*8.314*10-³*298]
=12.298kJ
∆G= ∆H-T∆S
=-12.978-298*0.044
=+0.134kJ/mol
Since ∆G is positive, the reaction is spontaneous
∆](https://image.slidesharecdn.com/unit-6-thermodynamics-220730192944-61c31657/85/unit-6-thermodynamics-ppt-14-320.jpg)
