TSRL Fact Sheet_Preclinical Accelerator_June 2016
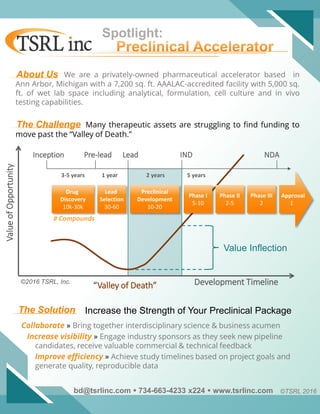
- 1. Inception Pre-lead Lead IND NDA 3-5 years 1 year 2 years 5 years ValueofOpportunity Development Timeline“Valley of Death” Value Inflection Drug Discovery 10k-30k Lead Selection 30-60 Preclinical Development 10-20 Phase I 5-10 Phase II 2-5 Phase III 2 Approval 1 ©2016 TSRL, Inc. # Compounds Spotlight: About Us We are a privately-owned pharmaceutical accelerator based in Ann Arbor, Michigan with a 7,200 sq. ft. AAALAC-accredited facility with 5,000 sq. ft. of wet lab space including analytical, formulation, cell culture and in vivo testing capabilities. Many therapeutic assets are struggling to find funding to move past the “Valley of Death.” Collaborate » Bring together interdisciplinary science & business acumen Increase visibility » Engage industry sponsors as they seek new pipeline candidates, receive valuable commercial & technical feedback Improve efficiency » Achieve study timelines based on project goals and generate quality, reproducible data The Challenge The Solution Increase the Strength of Your Preclinical Package bd@tsrlinc.com 734-663-4233 x224 www.tsrlinc.com ©TSRL 2016
- 2. NIAID SBIR Awardees 2015, 2014, 2013, 2011, 2010 Spotlight: Advancing lead compounds in infectious diseases Open up preclinical funding opportunities About Us- TSRL, Inc. is a preclinical accelerator based in Ann Arbor, Michigan. Our mission to advance promising compounds for treating severe and multi-drug resistant infections. Our R & D Capabilities: LC/MS/MS, ELISA, HPLC-UV analysis Chemical & metabolic stability Metabolite identification (ID) Tissue distribution Pharmacokinetics Permeability Formulation Efficacy models Exploratory toxicology BD & Marketing Capabilities: Competitive analysis Commercialization plan writing Pitch deck formation Website management Social media management bd@tsrlinc.com 734-663-4233 www.tsrlinc.com Small Business Research Grants (SBIR/STTR) » To foster scientific collaboration between small business concerns and research institutions » NIAID-approved waiver topics include novel anti-infective drugs » Phase I: ≤$300k/yr for two yrs; Phase II: ≤$1m/yr for three yrs NIH Research Grants – Omnibus Solicitations, BAAs and More! R01 » Supports specific projects within interests of investigator » ≤$500k direct costs/yr + ≤40% indirect costs for up to five yrs » Ex: Partnerships for Countermeasures Against Select Pathogens R21 » Supports new, exploratory and developmental research projects » ≤$275k direct costs/yr + ≤40% indirect costs for up to two yrs
