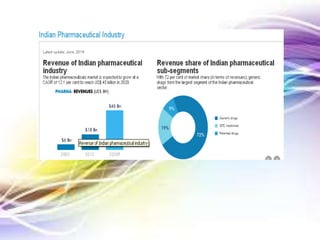
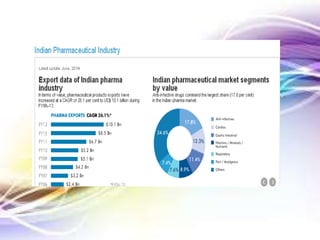
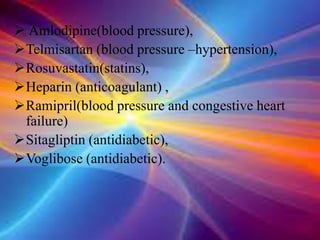
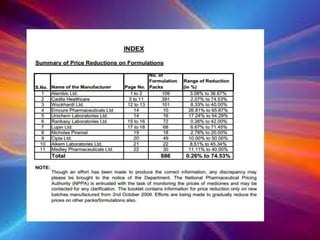
The document discusses the implications of the TRIPS agreement and the 2005 amendment to India's patent law on the pharmaceutical industry, highlighting both challenges and growth opportunities for generic drug manufacturers. It outlines the initial concerns regarding product patents hindering the industry, yet notes the significant increase in R&D investments and the growth of India's pharma market, projected to reach $45 billion by 2020. Additionally, it mentions recent drug price reductions and the necessary changes made to facilitate compliance with international patent standards.