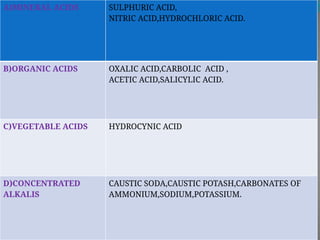
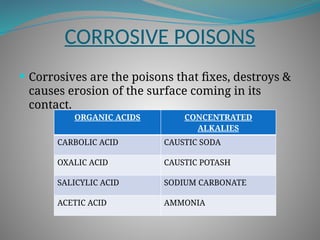
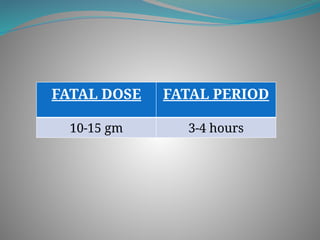
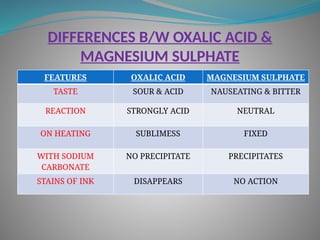
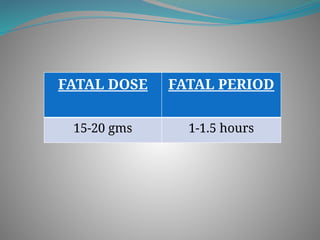
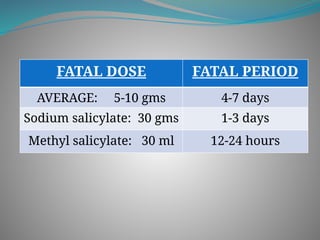
The document outlines various corrosive poisons, including organic acids and concentrated alkalies, detailing their properties, mechanisms of action, symptoms of poisoning, treatment, and medicolegal aspects. Specific compounds like carbolic acid, oxalic acid, and salicylic acid are discussed in terms of their toxicological effects, fatal doses, and autopsy findings. The document emphasizes the dangers of these corrosives, their classification, and the importance of proper treatment and understanding of poisoning incidents.