This document summarizes key concepts about reactions in aqueous solutions including:
1. Polar substances like water are able to dissociate ionic compounds into ions when dissolved due to the unequal distribution of charge in polar bonds.
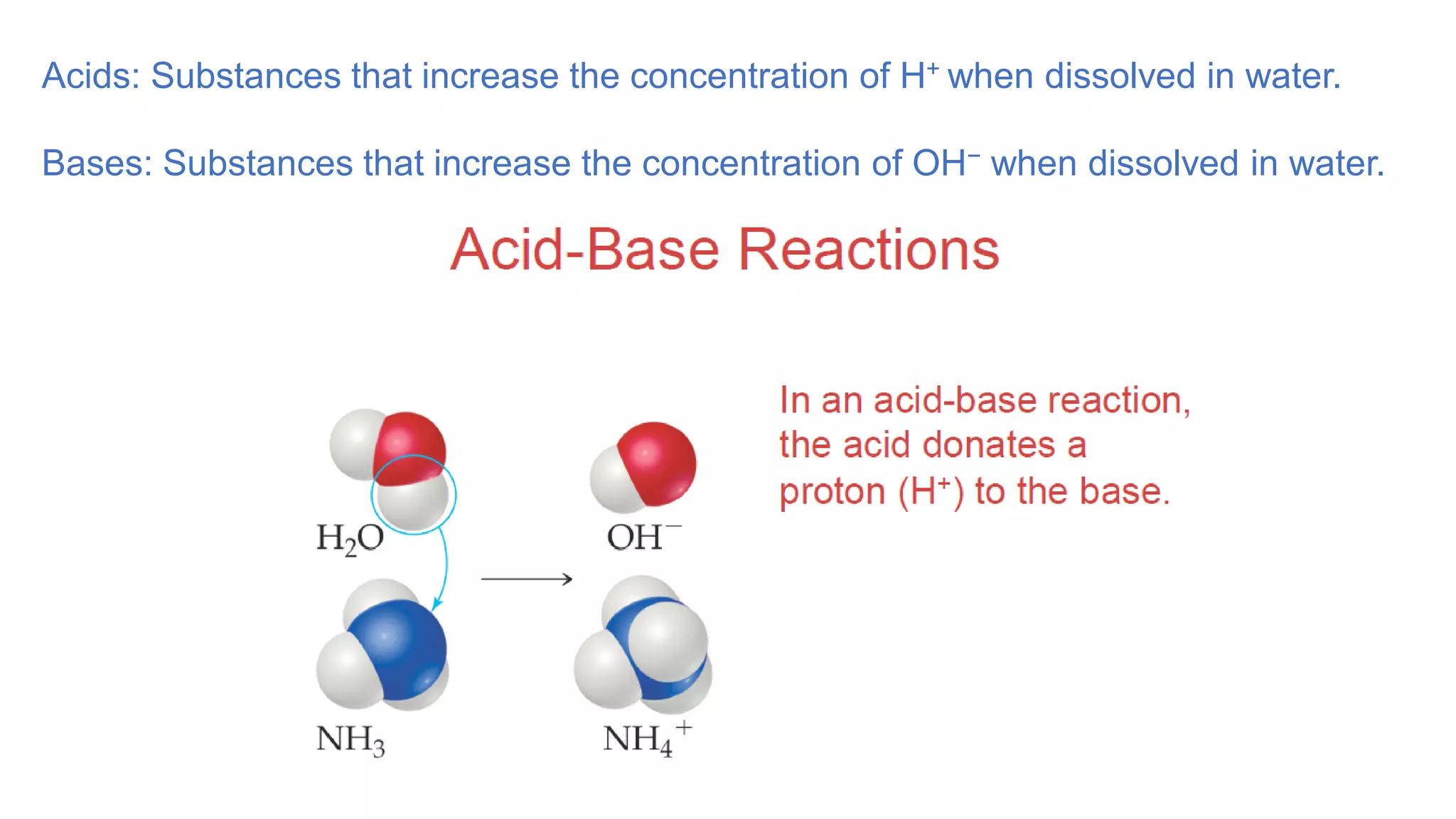
2. Substances that dissociate into ions when dissolved in water are electrolytes, while molecular compounds tend to be nonelectrolytes except for acids and bases.
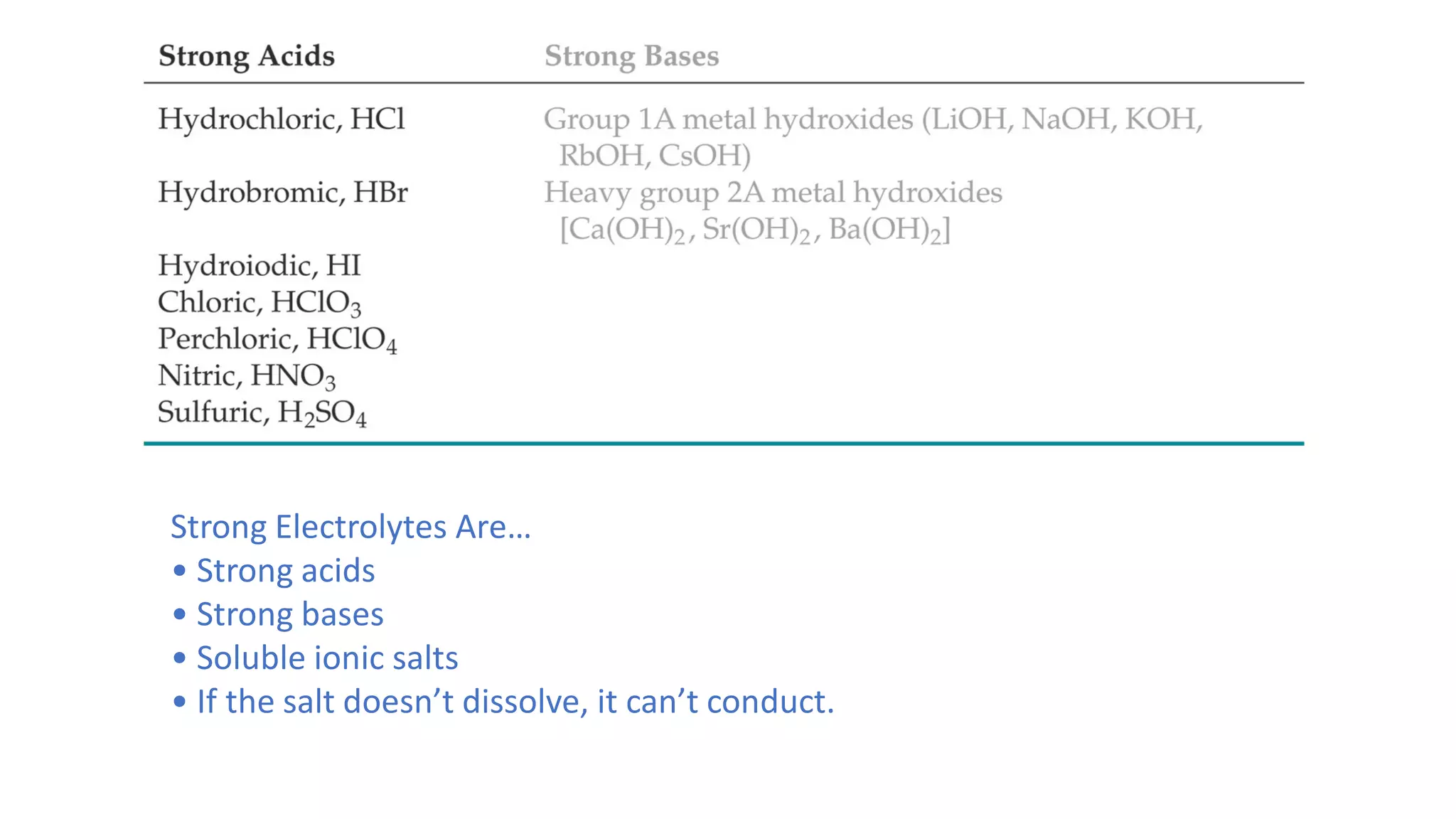
3. Strong electrolytes include strong acids, strong bases, soluble ionic salts, and substances that conduct electricity when dissolved in water.