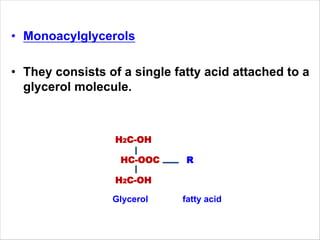
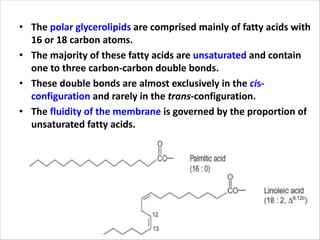
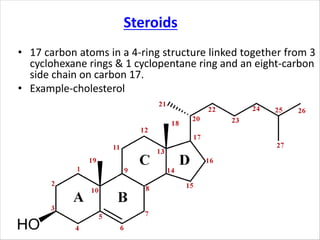
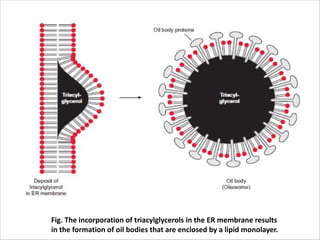
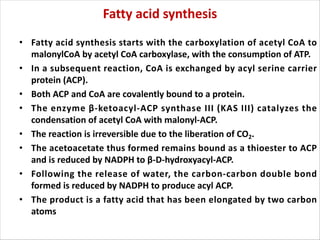
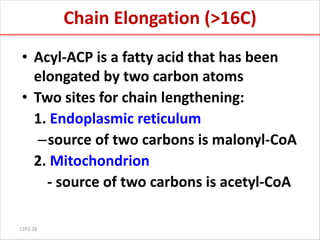
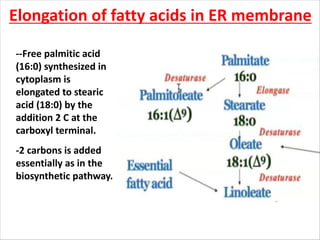
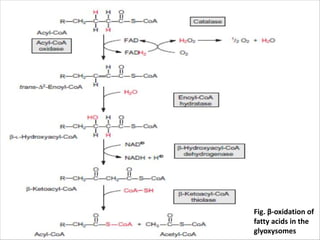
Lipids are fatty acids and their derivatives that play important structural and storage roles in plants. There are three main types of lipids - glycerolipids, sphingolipids, and steroids. Glycerolipids like mono-, di-, and triacylglycerols are the main constituents of plant membranes and storage lipids. Fatty acids are synthesized in plastids from acetyl-CoA and undergo elongation and desaturation. Glycerolipids are then synthesized in plastids or the endoplasmic reticulum. Triacylglycerols provide carbon and energy storage in seeds and are broken down by lipases during germination.