Embed presentation
Download to read offline



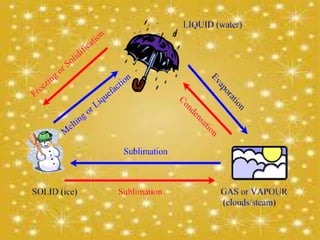







This presentation discusses phase transitions between different states of matter - solid, liquid, and gas. It defines phase transitions as transformations between states of matter through heat transfer, such as condensation (gas to liquid), evaporation (liquid to gas), freezing (liquid to solid), melting (solid to liquid), deposition (gas to solid), and sublimation (solid to gas). It also provides brief definitions and examples of solids, liquids, and gases as different states of matter.










