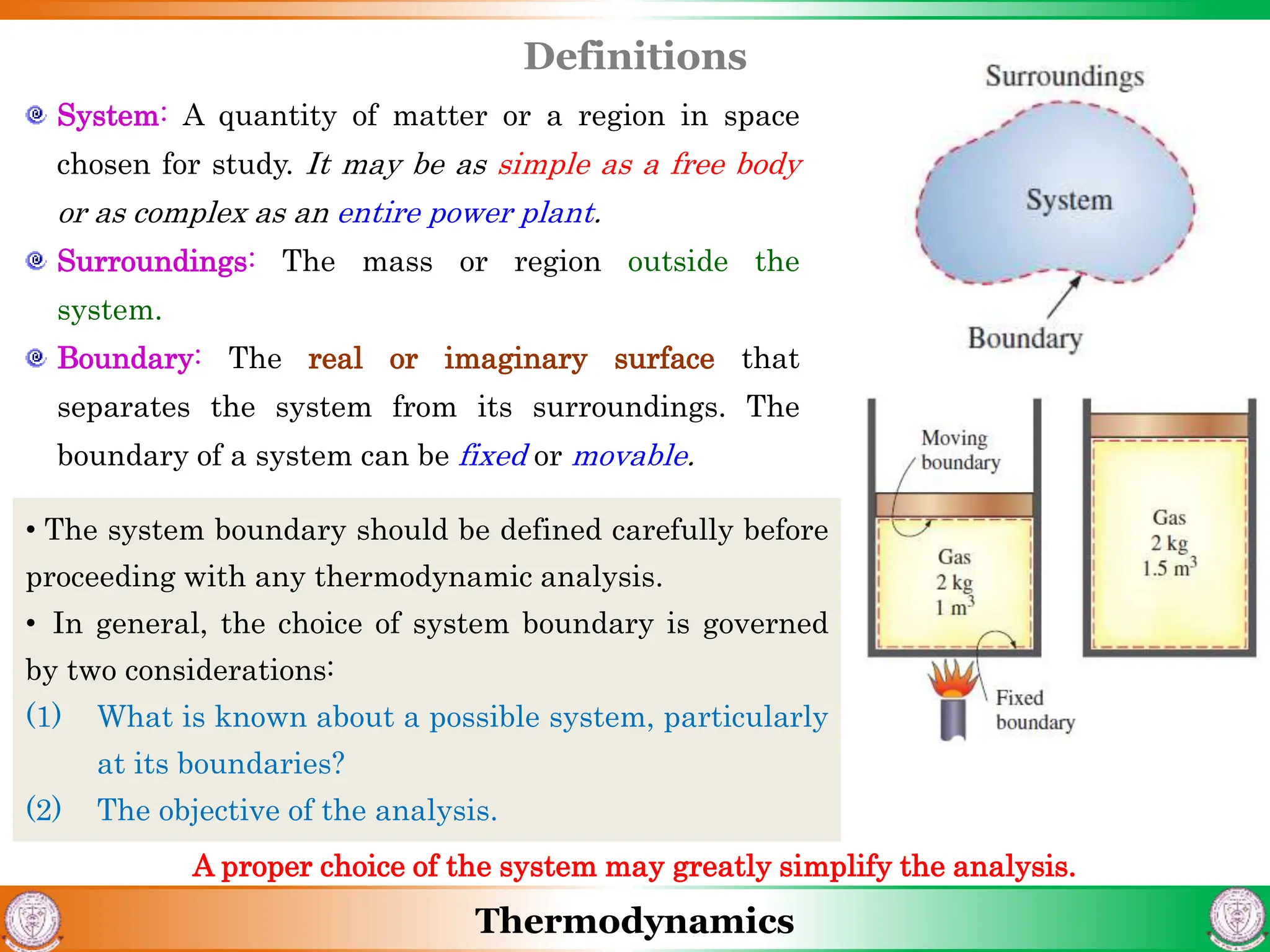
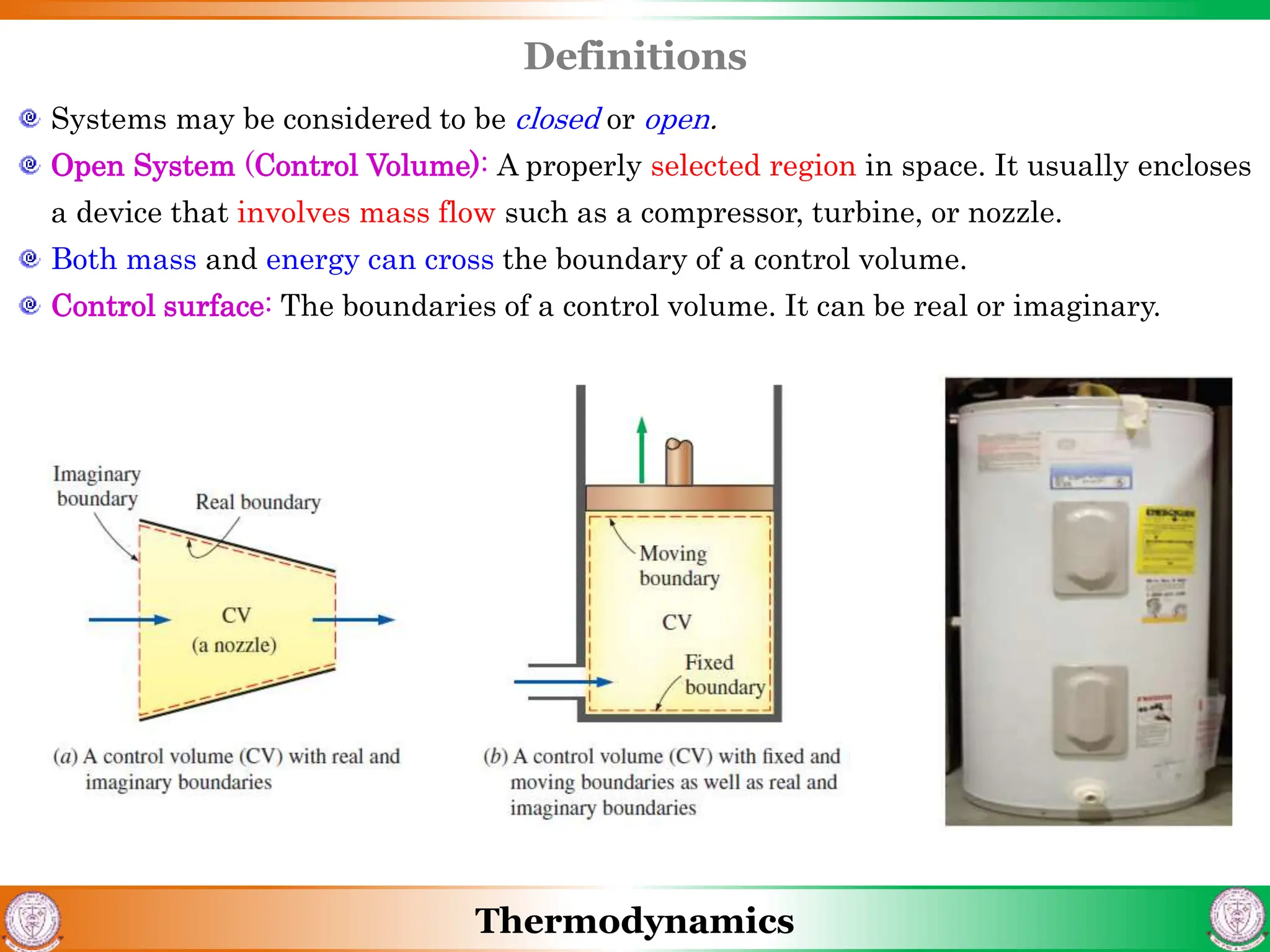
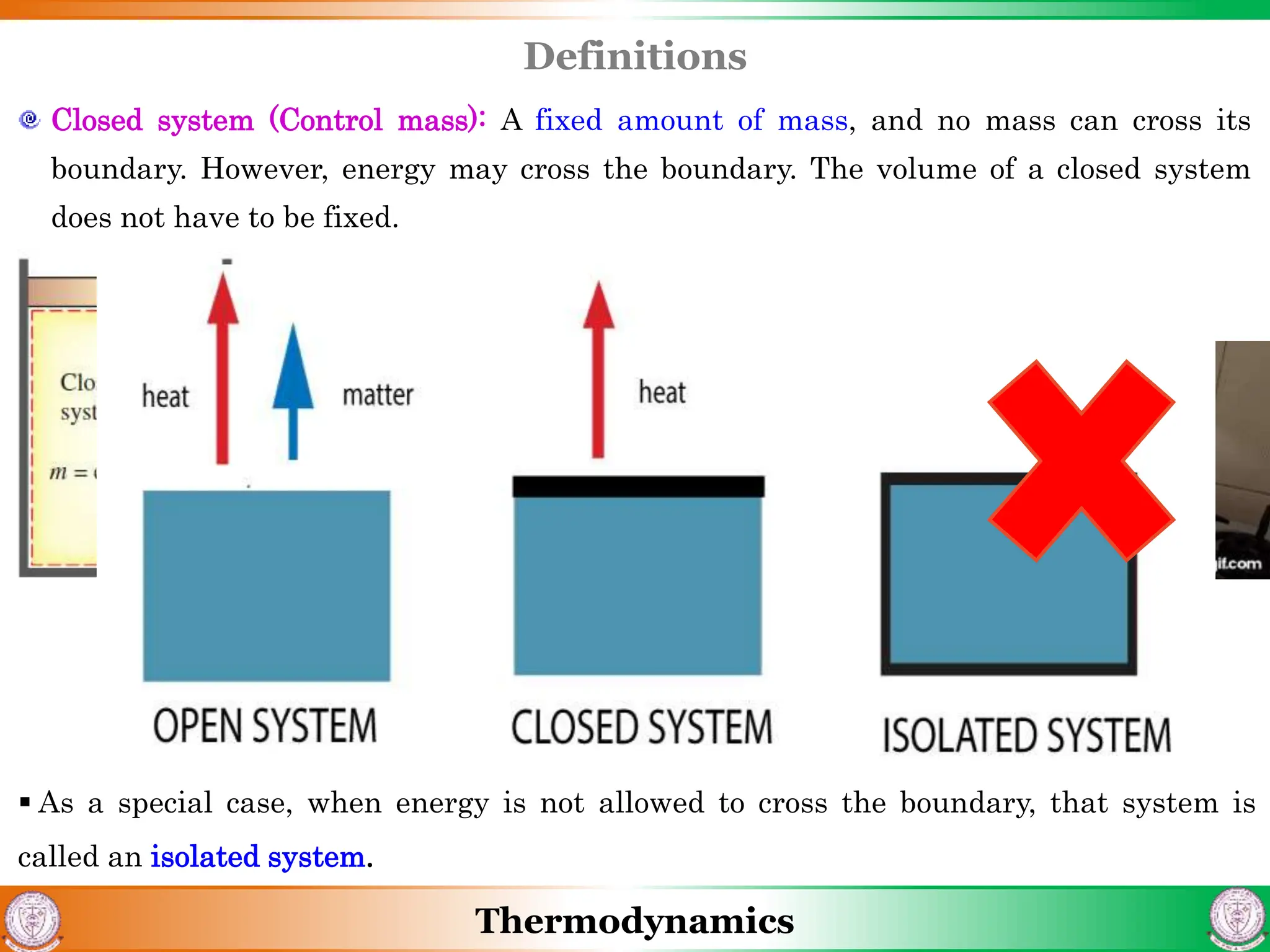
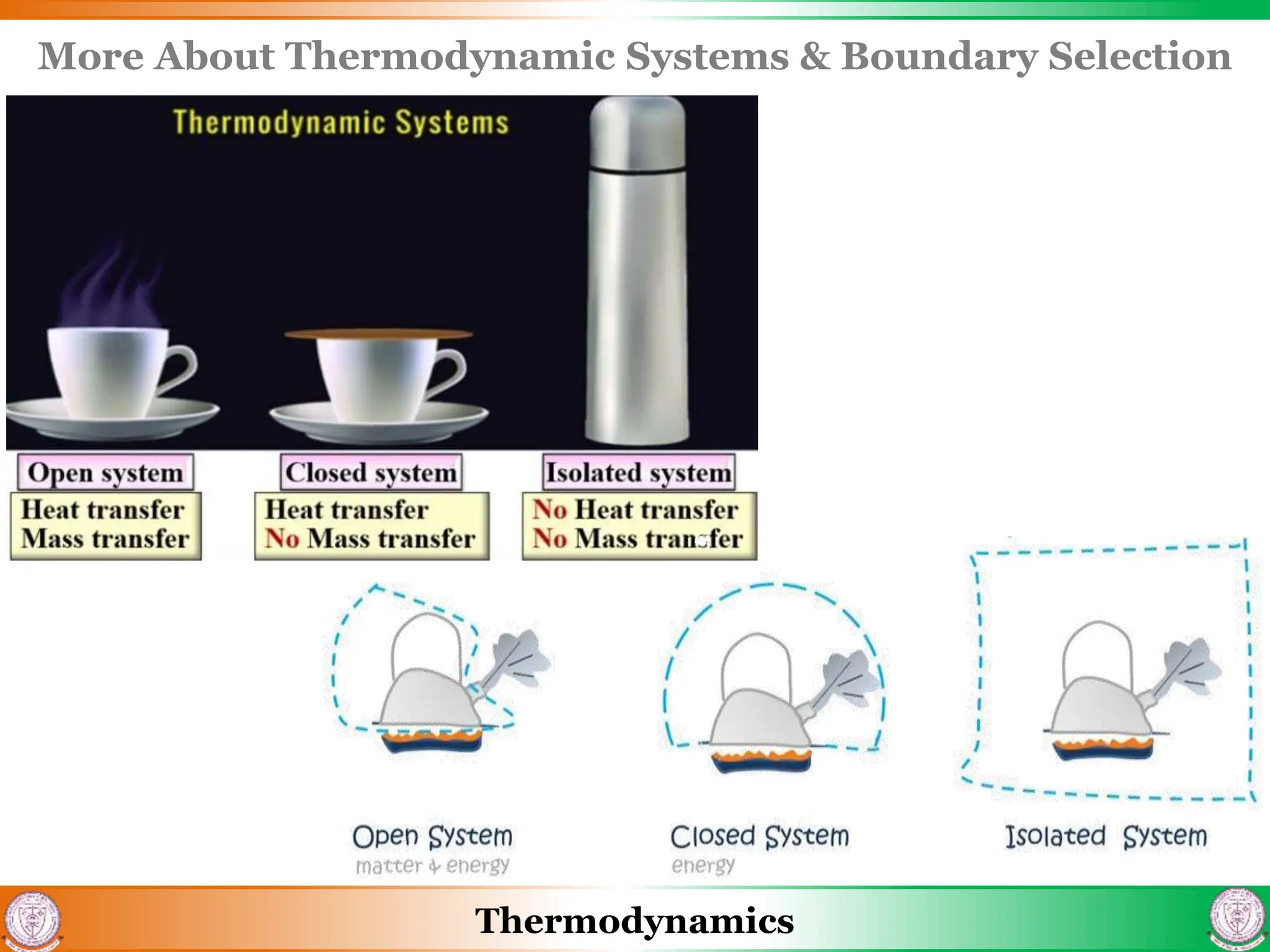
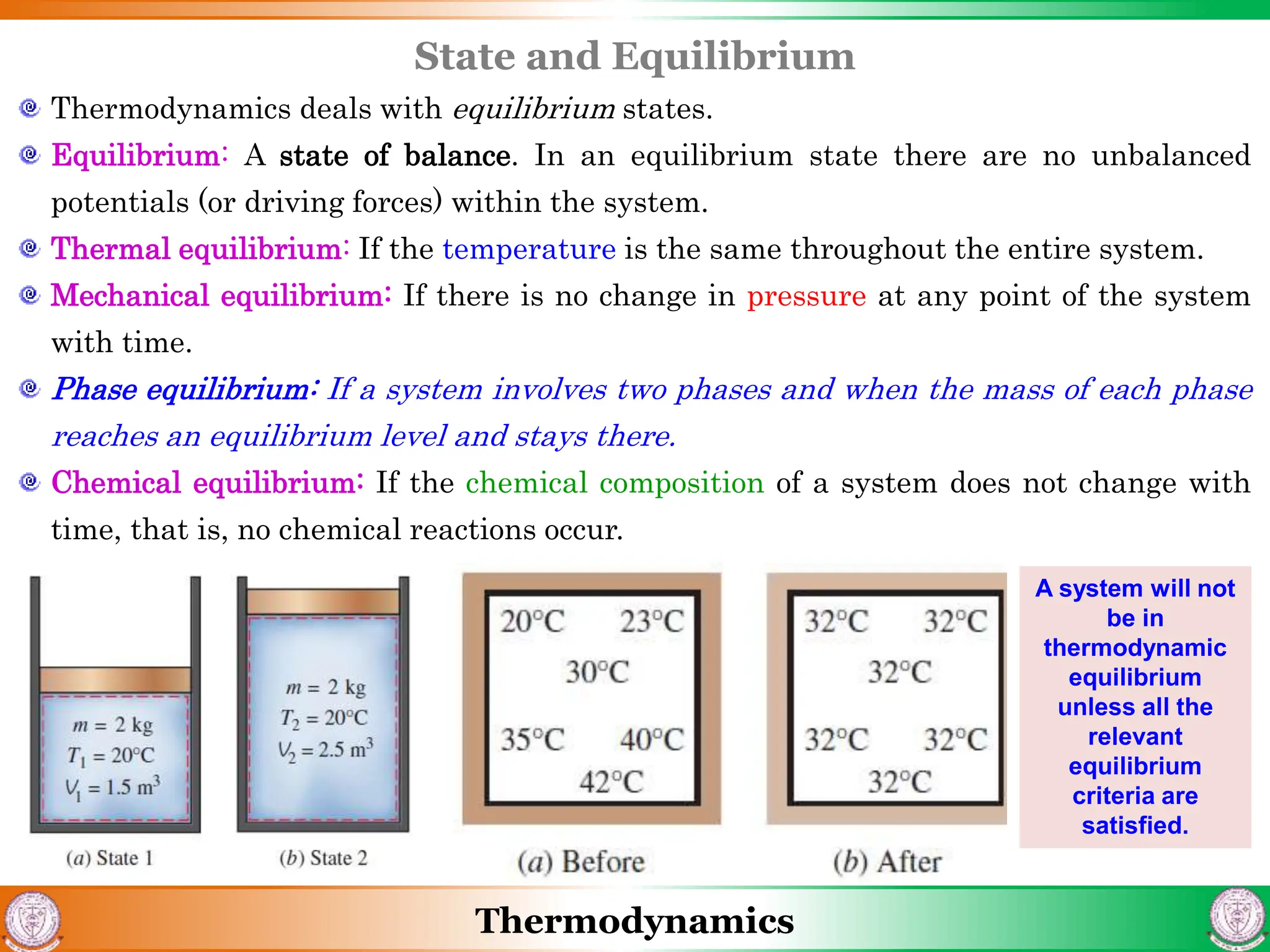
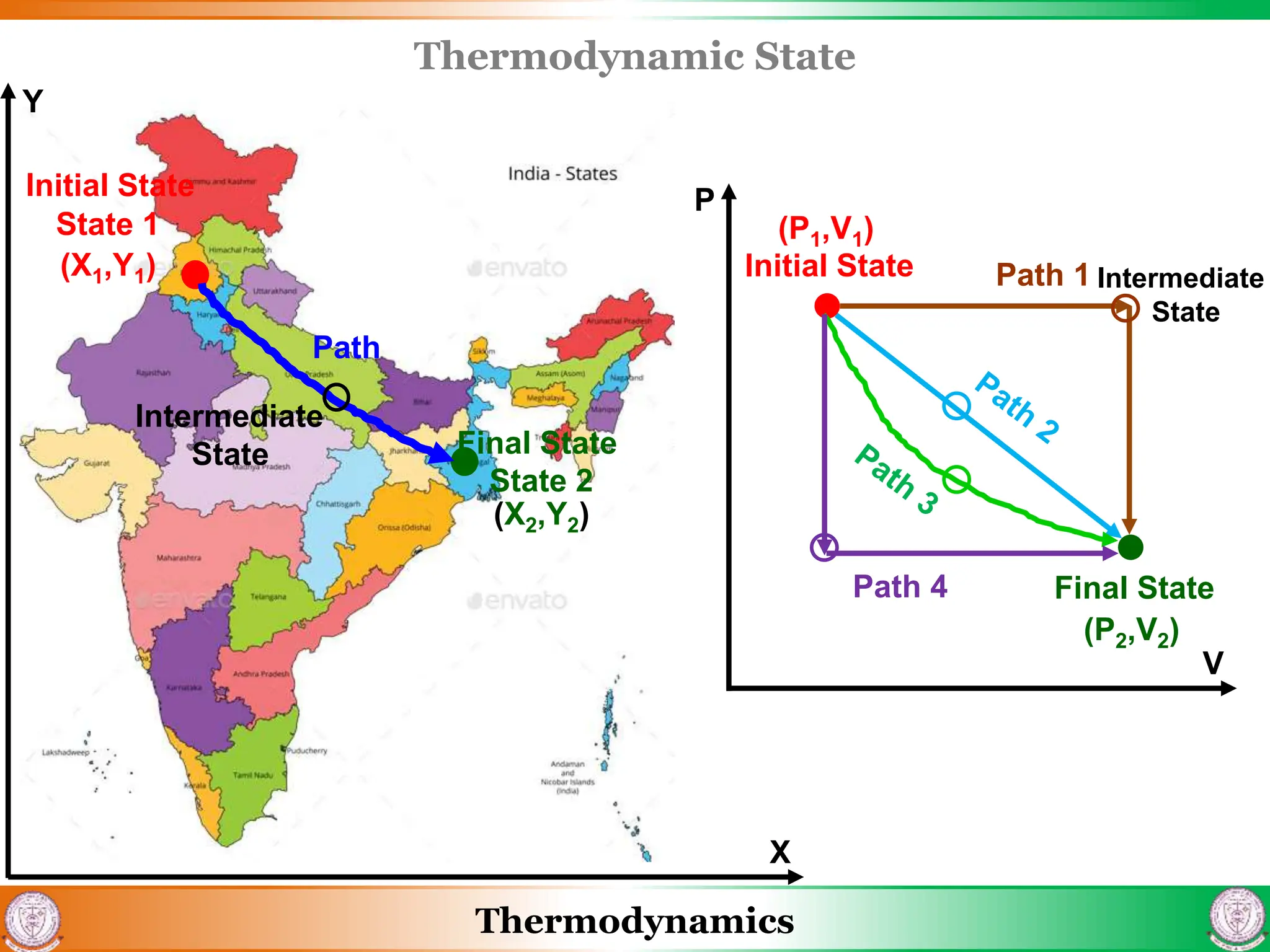
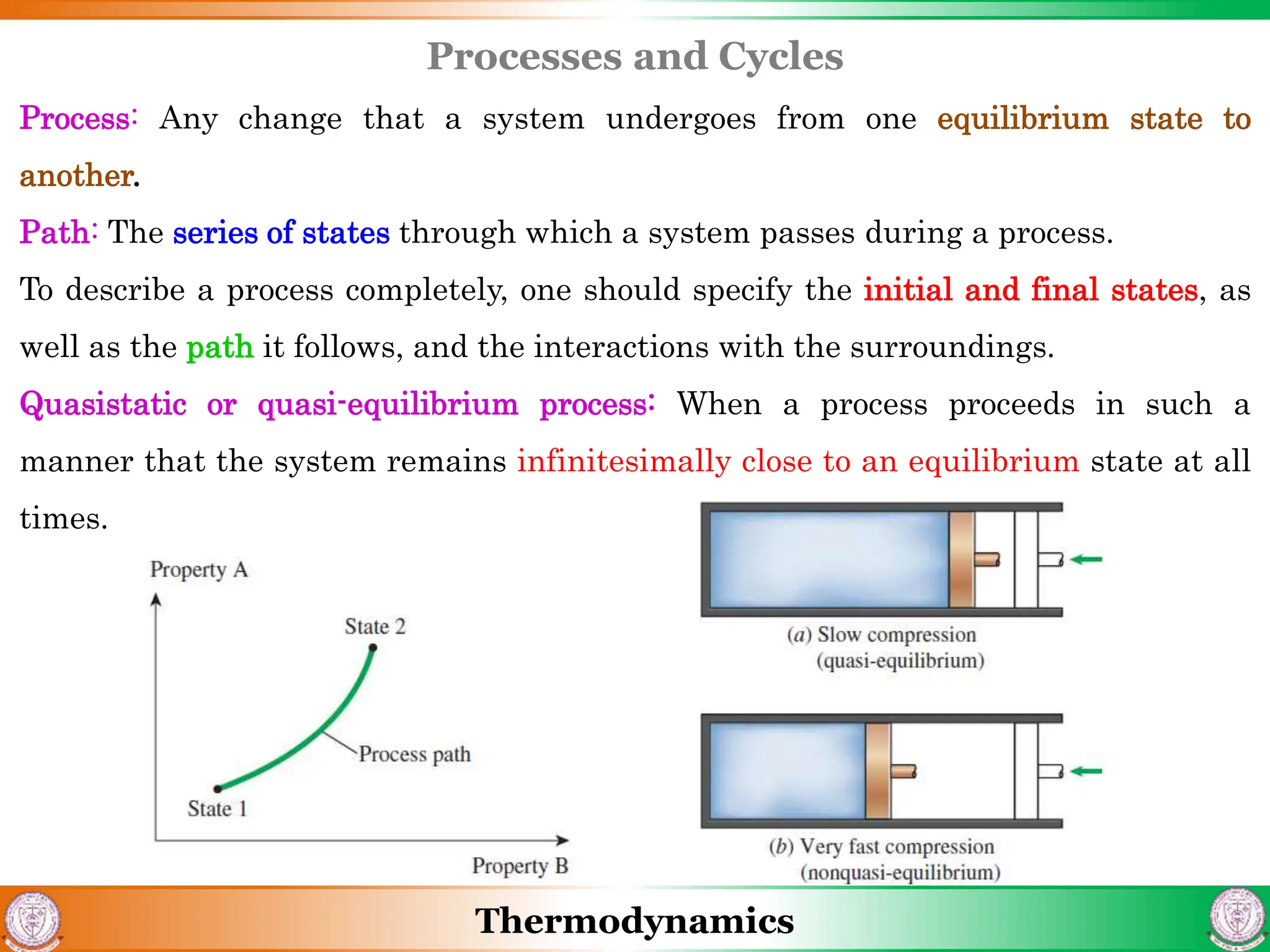
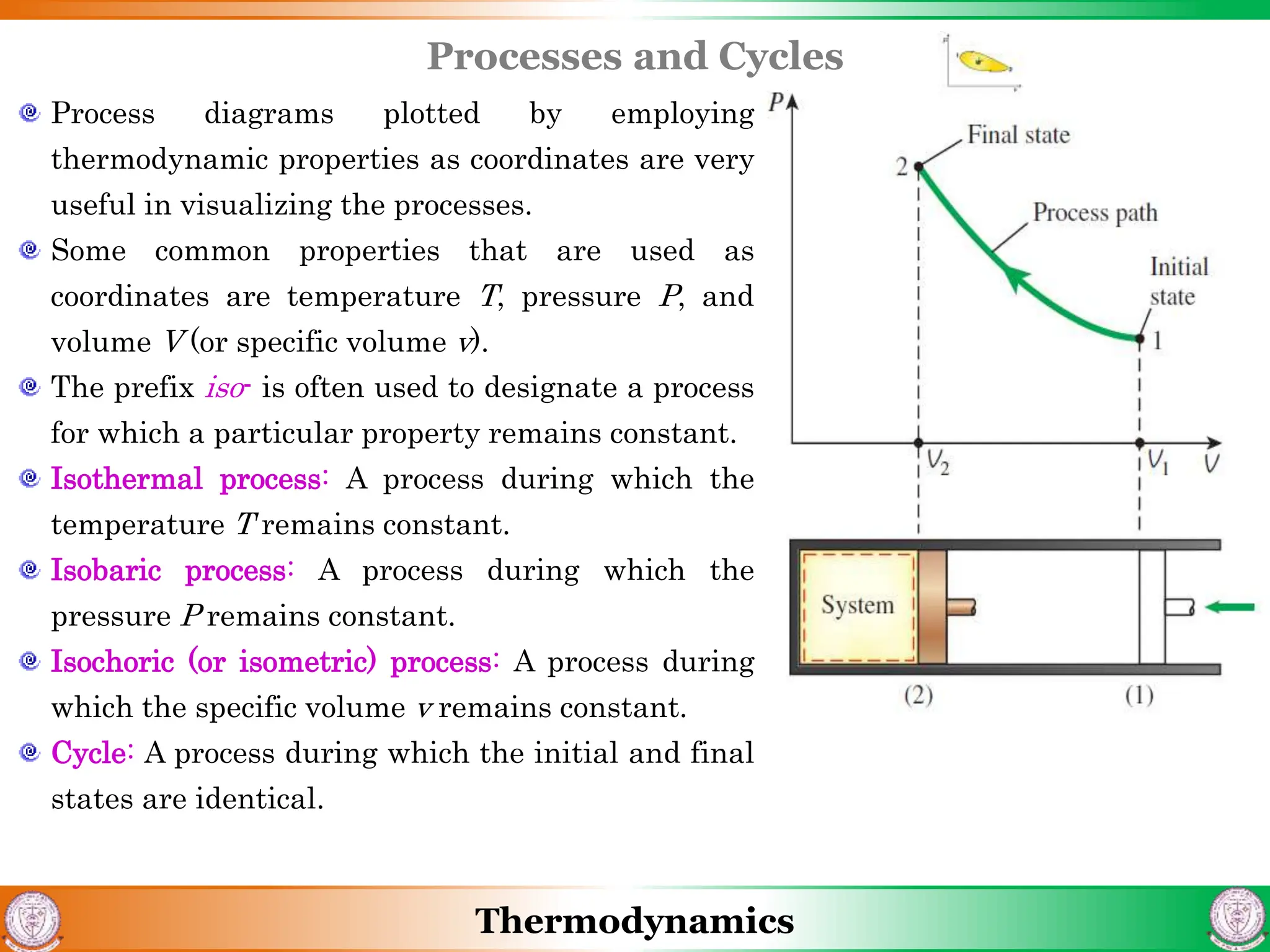
1) The document discusses basics of thermodynamics including definitions of key terms like system, surroundings, boundary, state, process, and cycle.
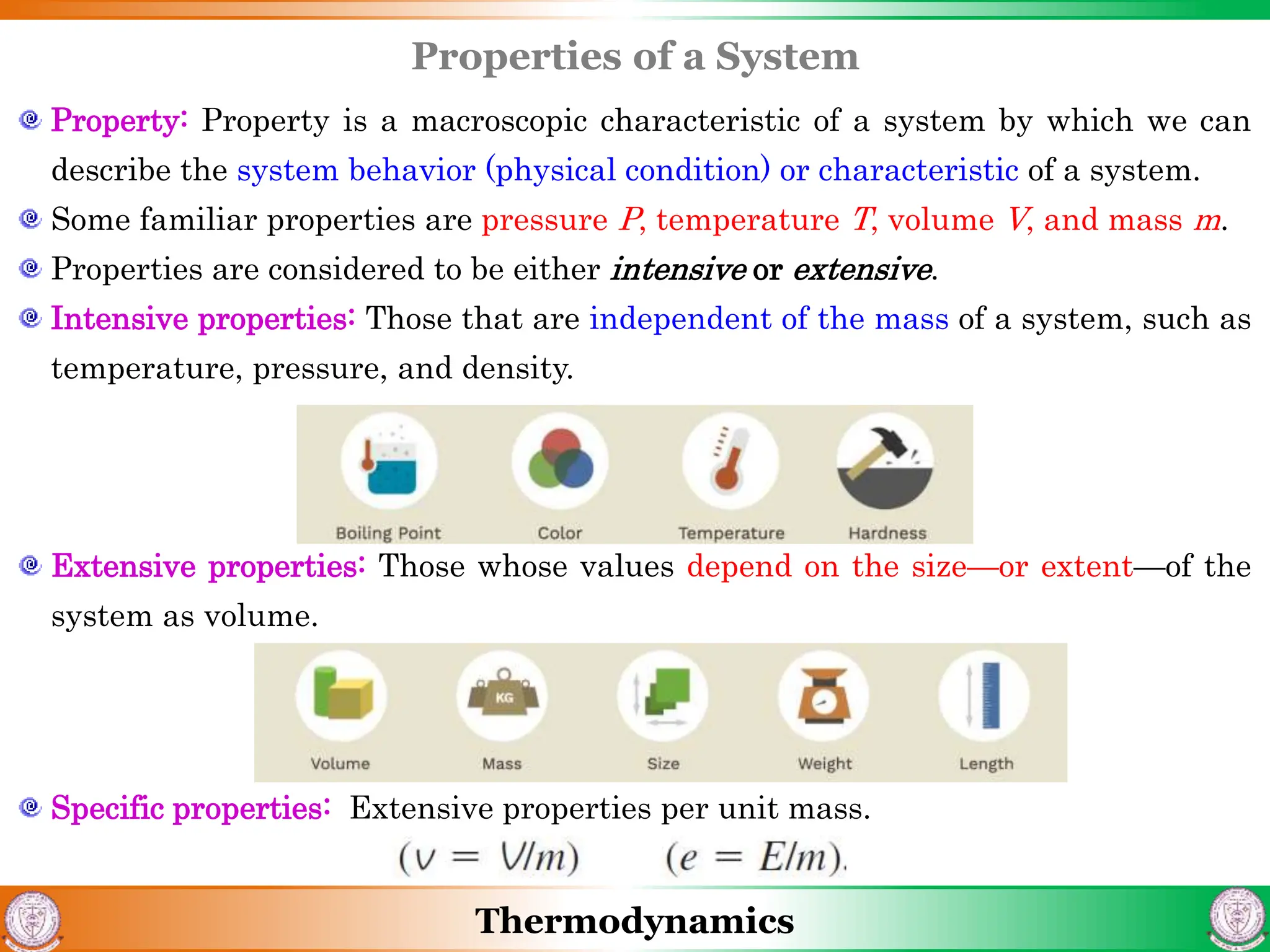
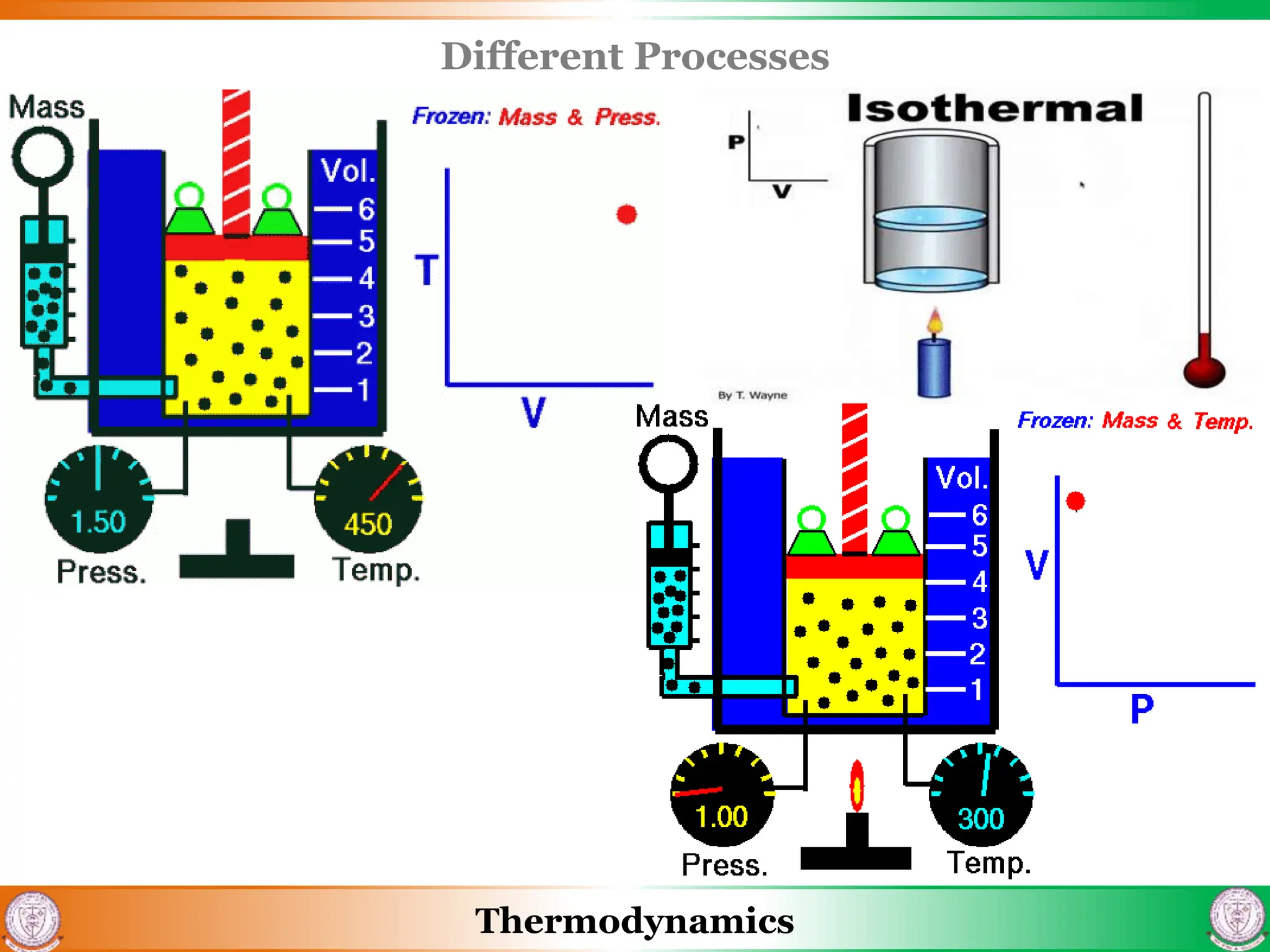
2) It covers concepts of extensive and intensive properties, equilibrium states, and different types of processes like quasistatic, isothermal, isobaric, and isochoric.
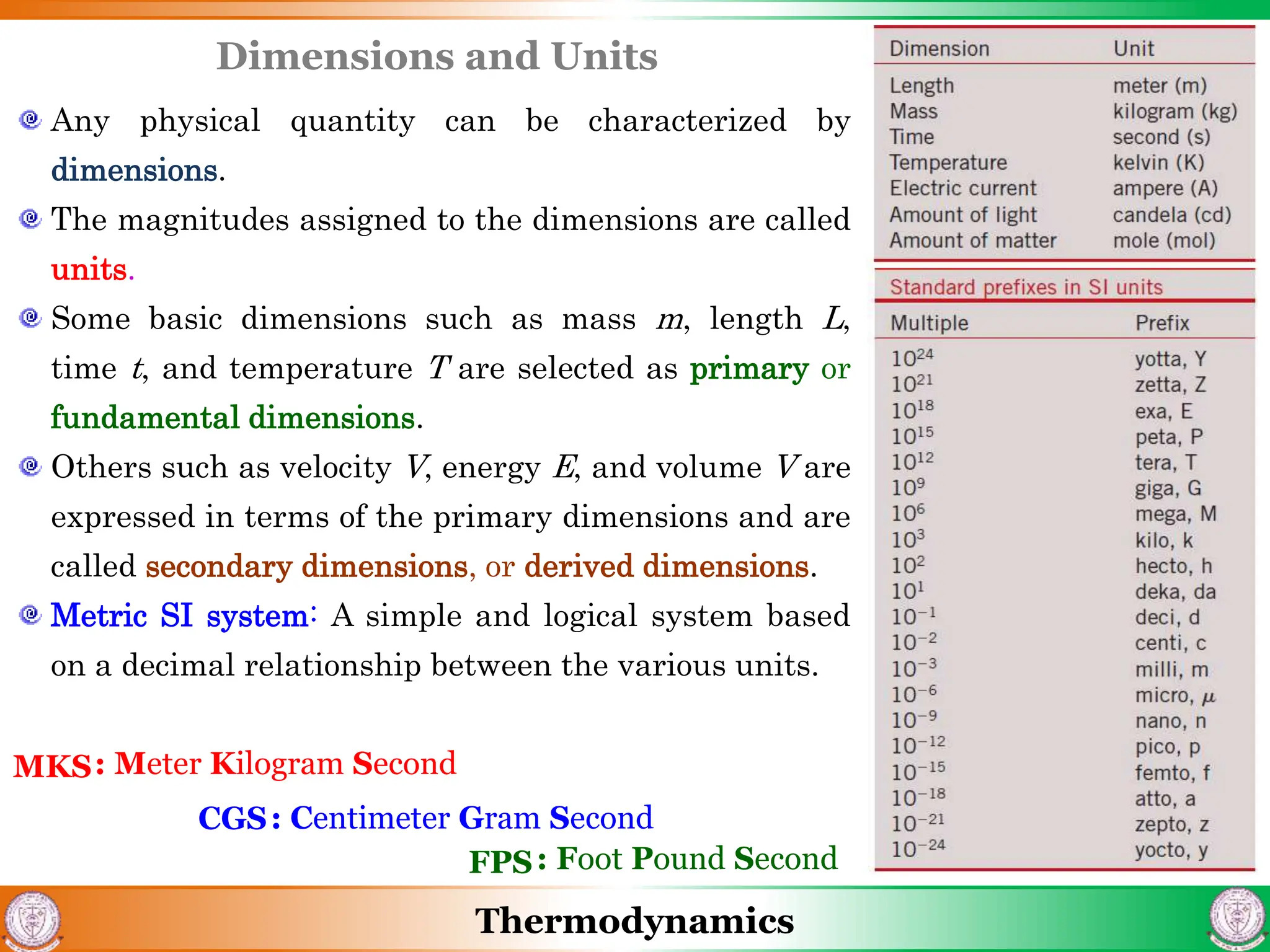
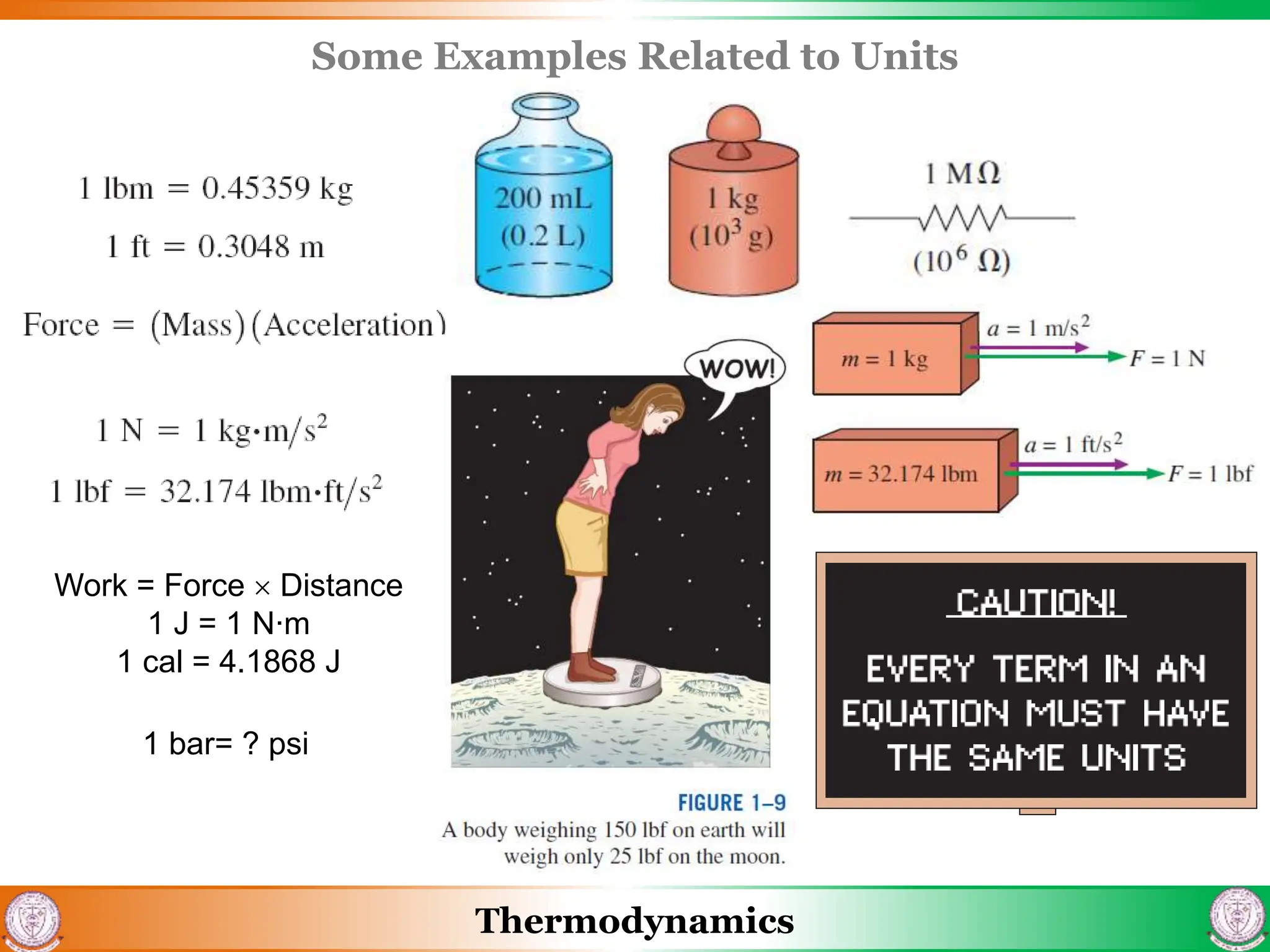
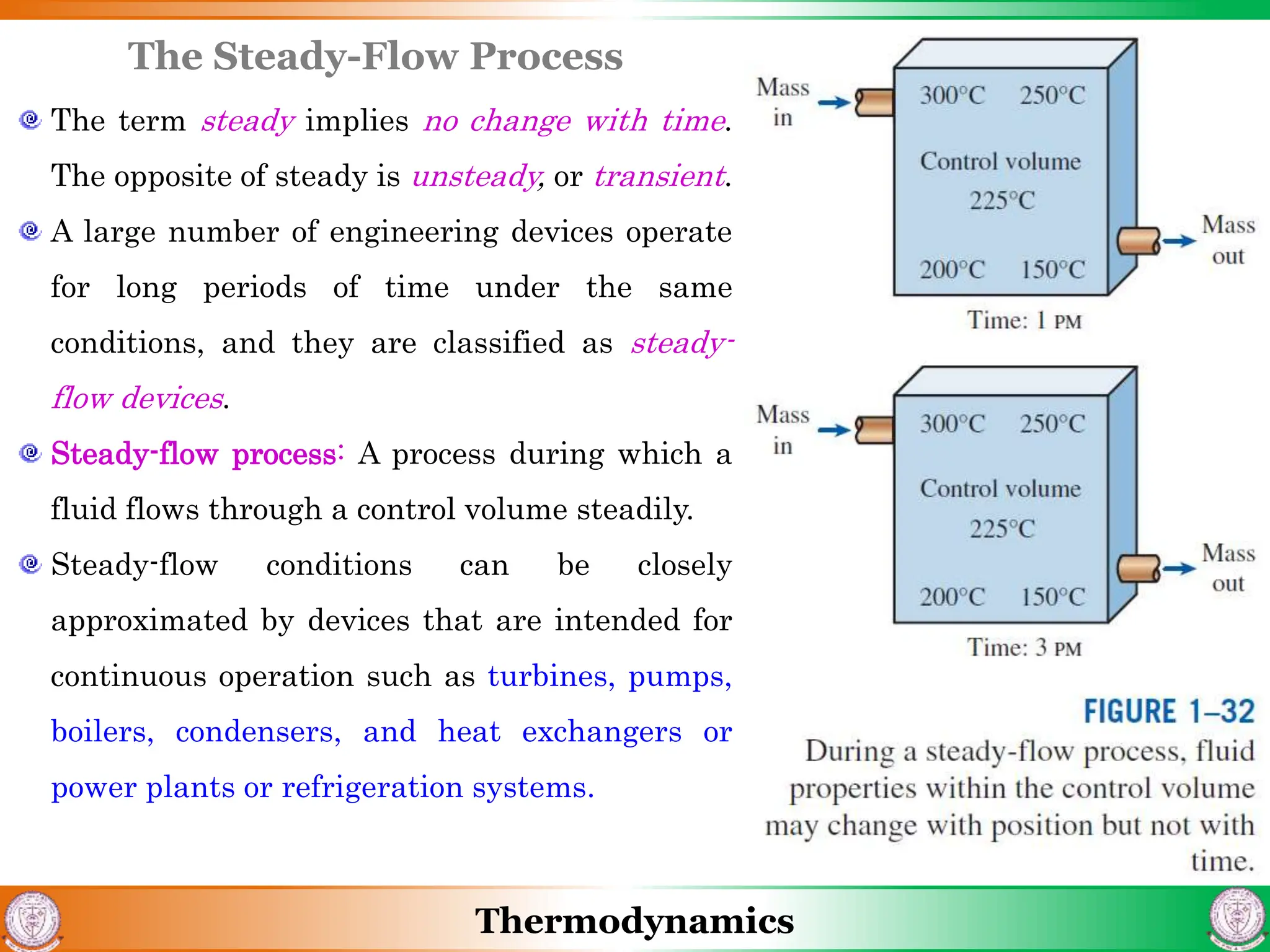
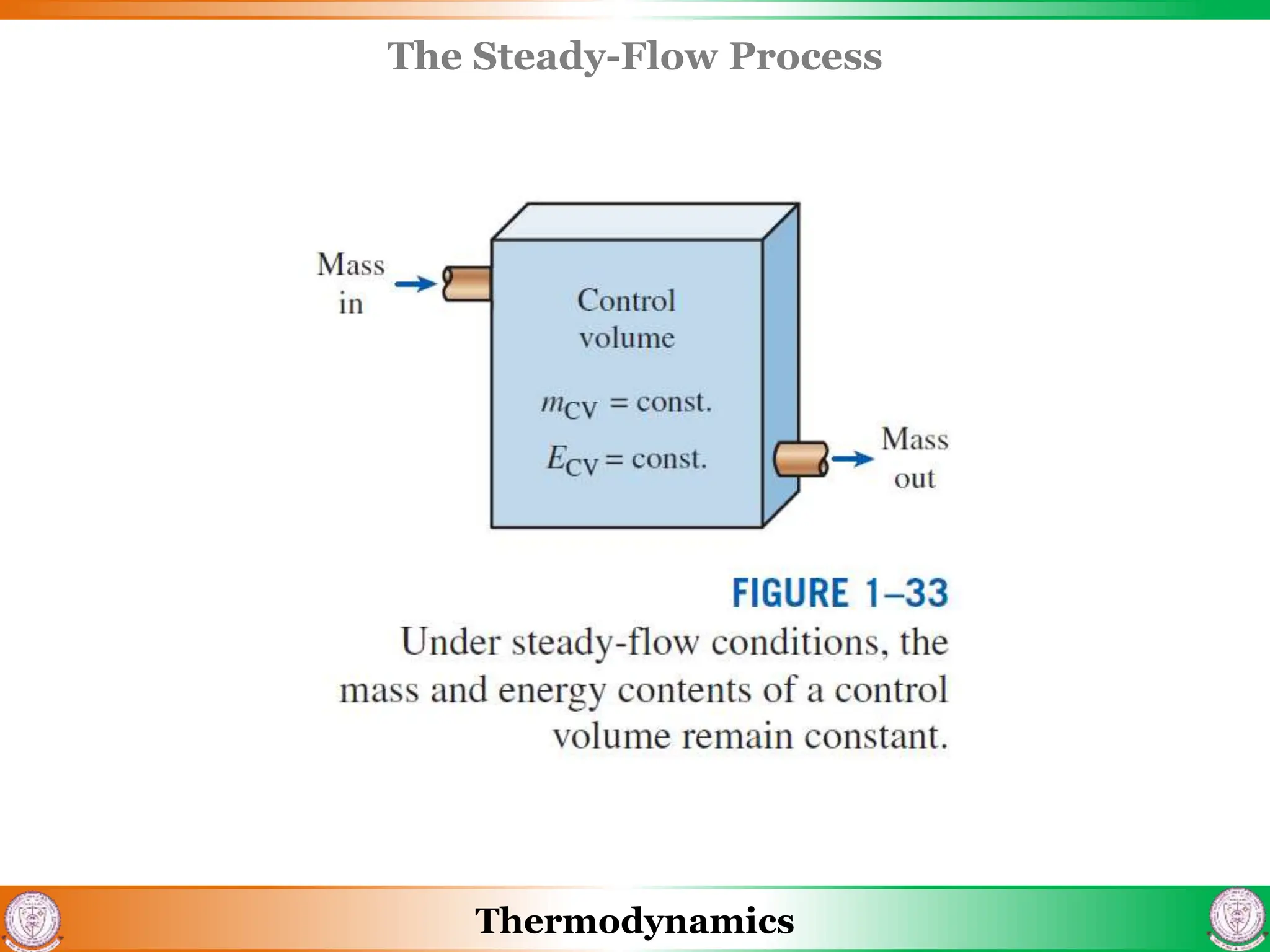
3) The document also discusses steady flow processes, units and dimensions in thermodynamics, and provides examples of applying concepts to engineering problems.