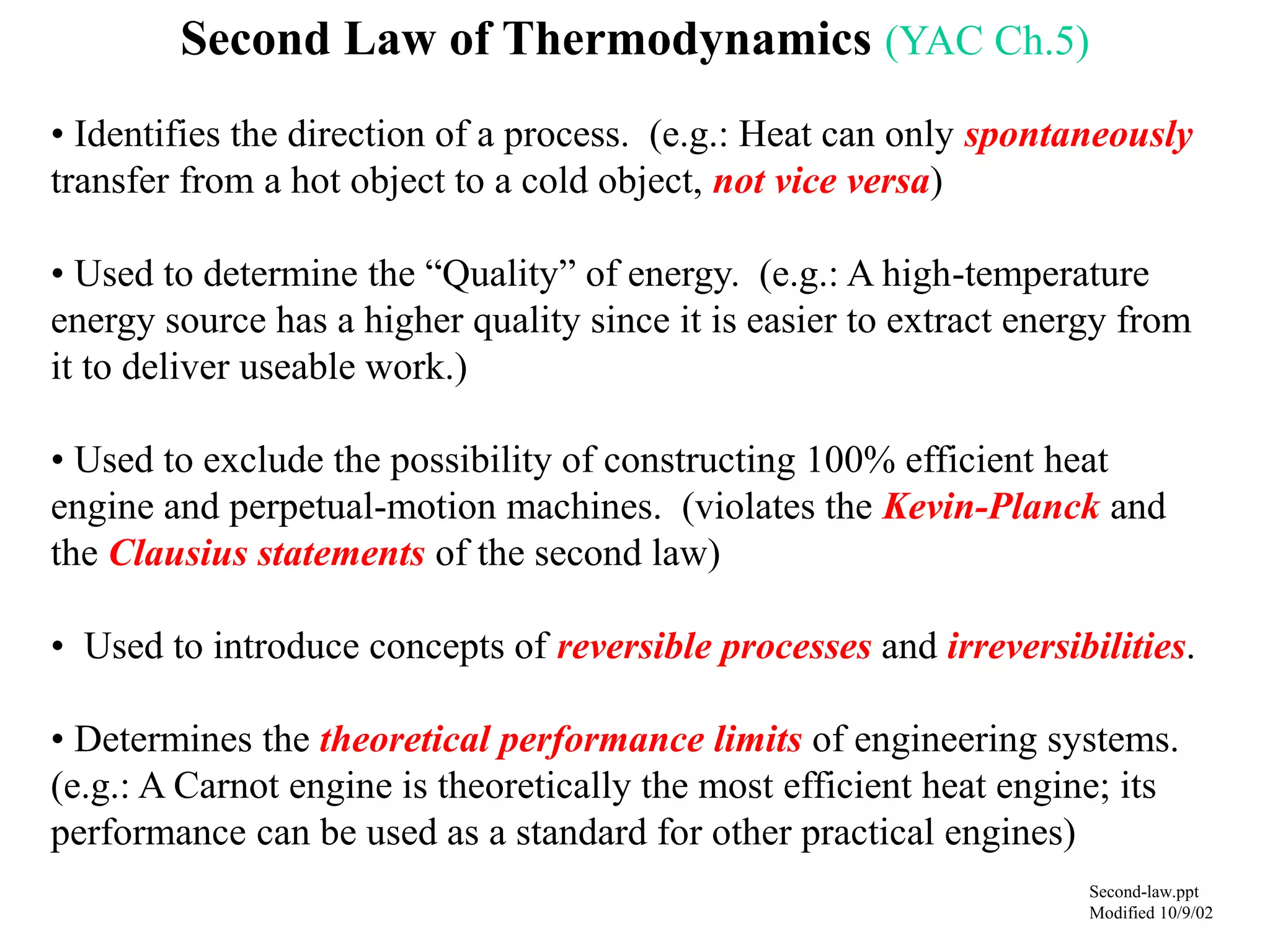
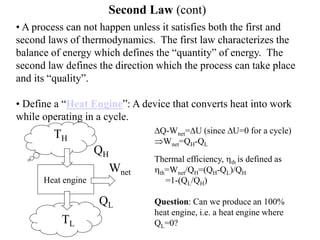
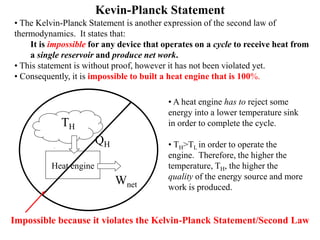
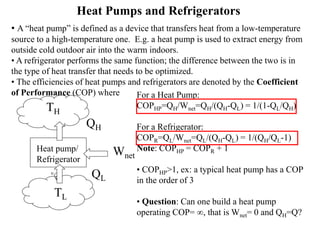
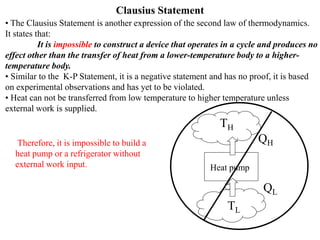
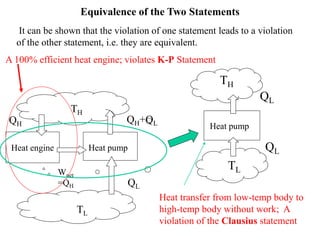
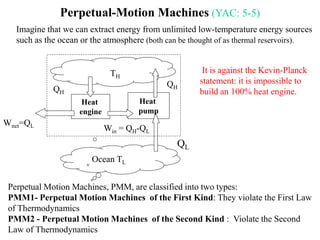
The Second Law of Thermodynamics identifies the direction of processes like heat transfer, determines the quality of energy sources, and excludes perpetual motion machines. It introduces concepts like reversibility and irreversibility. A process must satisfy both the first and second laws. The Second Law is expressed by both the Kelvin-Planck statement, which prohibits a heat engine from receiving heat from a single reservoir and producing work without rejection, and the Clausius statement, which prohibits heat transfer from a cold to hot body without work. Real processes are irreversible but reversible processes are theoretically most efficient.