1. The author taught a lesson on ionic and covalent bonding to a class of 26 high-achieving Year 9 students.
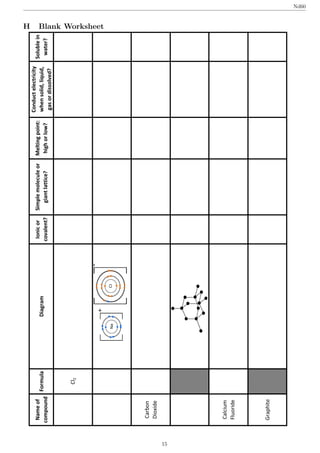
2. During the lesson, the students completed a worksheet in pairs to consolidate their understanding of the differences between ionic and covalent bonding and how this relates to substance properties.
3. An evaluation found that most students understood the concepts well after the lesson, though a later quiz showed some students still struggled with identifying mistakes in example dot-and-cross diagrams.
![Materials Education Report
Teaching and Revision of Chemical Bonding
Nd60
April 23, 2014
Abstract
The concepts of ionic and covalent bonding underpin the teaching of iGCSE chemistry. Students often find it difficult
to remember and rationalise the various properties, and present their answers in the best way to achieve top marks.
I chose to use my teaching time to focus on highlighting the differences between these types of bonding and how this
manifests in the substance properties.
1 Background
I taught at The Perse School. The Perse is an indepen-
dent, selective, co-educational school which consistently
achieves very high grades. Last year 93% of the 145 iGCSE
chemistry students achieved an A/A*; 100% achieved A*
to C [1].
I taught a class of 26 Year 9 students who had just
started their iGCSE chemistry course. Although the class
were nominally a mixed ability class, the students were
generally A/A* students in accordance with the demo-
graphic of the school. The class were arranged in a seat-
ing plan which generally put weaker students near helpful
high-achieving students, and kept louder students apart.
I based my teaching on the Edexcel specification for
ionic and covalent bonding - see appendix A. I observed
the first lesson and then acted as a teaching assistant in
most of the others. In my fourth lesson I taught a 20
min section on covalent bonding, and in my sixth lesson I
taught a 1 hr 20 mis revision session to consolidate knowl-
edge of ionic and covalent bonding, structure and proper-
ties.
2 Previous Treatment of the Topic
The Perse provides teachers with a Scheme of Learning
as a framework (see appendix B). The exact methods and
order of delivering material is left to each teacher’s discre-
tion.
Previously Dr Khimyak had taught dot-and-cross di-
agrams by showing students examples on the board and
then giving them a worksheet. She also had not previously
pulled together properties and examples of ionic and co-
valent substances into one table (see section 5.2), which I
chose to do.
3 Project Topic
From my previous experience of tutoring ionic and cova-
lent bonding and a discussion with Dr Khimyak we de-
cided that I could focus on the confusion between the dif-
ferent types of bonding. Often students find it difficult
to justify the properties exhibited by compounds, espe-
cially when referring to intra- and inter-molecular bond-
ing. There is also confusion about the relative strength of
bonds (ionic or covalent) and intermolecular forces (such
as van der Waals’ forces).
For my special project I ran a revision session on
these topics. The objective was to solidify their knowl-
edge through a mixture of class discussion, a worksheet
which they completed in pairs and a review of the work-
sheet. This worksheet was designed to also act as a good
revision resource for their upcoming test, and eventually
for their iGCSE exams.
I considered two other options before settling on a work-
sheet oriented lesson: a dictated class and an interactive
class. From observation of previous lessons I had seen how
quickly the class lost focus whenever they had to copy
down dictated notes. Although this would leave them with
a good resource it would not improve their knowledge. A
more interactive lesson would engage the class however it
would be more difficult to leave them with useful revision
material. The class, and particularly the weaker students,
were also likely to become less focused if given more scope
for conversation. Thus I decided a worksheet was the best
option.
4 Planning
In appendices C & D I have included lesson plans for the
two lessons I taught.
The first lesson was a short 20 min section on covalent
bonding. I prepared templates of covalent molecules, such
as those shown in appendix E. I gave the 13 listed on the
specification to each table, along with coloured counters to
represent electrons. They used these to decide where the
electrons went, check with me or Dr Khimyak and then
draw it out in their exercise books.
For the special project lesson I prepared two online
quizzes: one to be taken at the beginning of the lesson
and one at the end. These are included as appendices F
& G and were made and run using the online resource
Socrative [2]. The main section of the lesson was based on
filling in a worksheet. I have included the blank copy given
to the students and the filled copy I used as appendices H
& I.
1](https://image.slidesharecdn.com/f7055ef5-27a7-4888-9d7d-2b64e5cc34b1-150827133551-lva1-app6892/85/TeachingReport-1-320.jpg)


![REFERENCES Nd60
0
20
40
60
80
100
1 2
PercentageScore/%
Quiz
Boxplot with Quiz 1 Scaled
Figure 4: Boxplot indicating the spread of data in the class
for each quiz after Quiz 1 was scaled
3. How well they would retain the information and un-
derstand the material weeks or months later
In my special project I aimed to improve the students’
understanding at the time of the lesson whilst also ensur-
ing the material would be better understood in the future,
and to provide them with a resource to help with this.
From observation of the students I found that they were
most engaged and learned most when they were actively
working as it gave them time to consider the problem
themselves.
On reflection the results indicate an improved under-
standing of the topic after the lesson. I was not able to
test whether this lesson helped the students to retain the
information over a longer timespan.
References
[1] iGCSE level results by subject in 2013 - Year 11.
(2013)
Available: http://www.perse.co.uk/upper/curriculum/exam-
results/. Last accessed 18th Mar 2014.
[2] Socrative. (2014)
Available: http://www.socrative.com/. Last accessed
18th Mar 2014.
4](https://image.slidesharecdn.com/f7055ef5-27a7-4888-9d7d-2b64e5cc34b1-150827133551-lva1-app6892/85/TeachingReport-4-320.jpg)